| J Neuromonit Neurophysiol > Volume 5(2); 2025 > Article |
|
Abstract
Notes
References
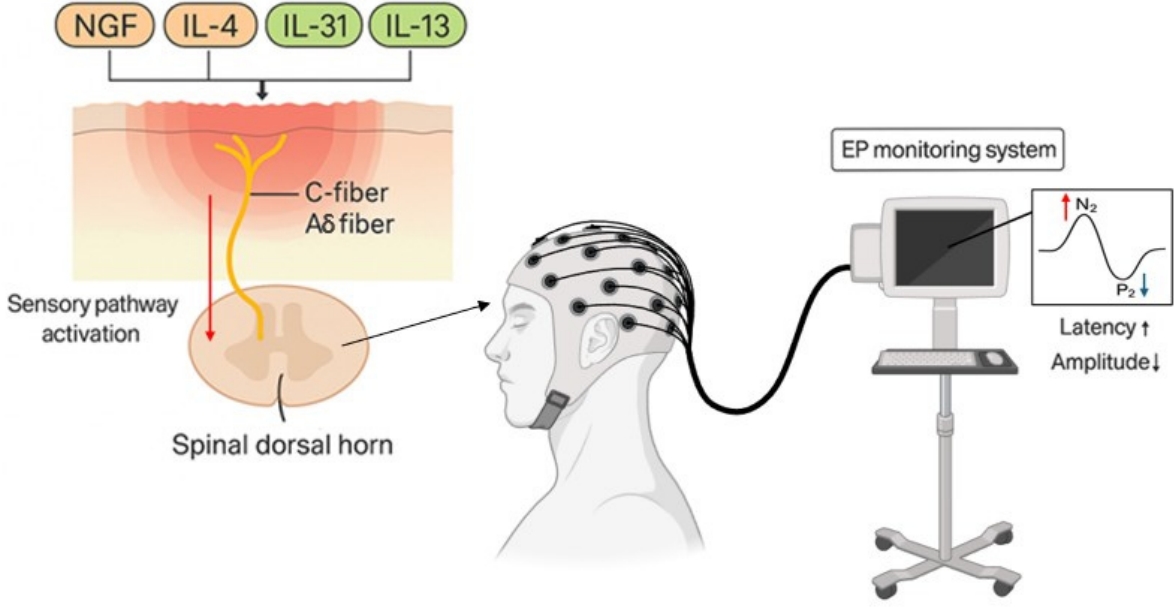
Figure┬Ā1.
Table┬Ā1.
| EP modality | Stimulation type | Target fiber(s) | Representative component | Analytic parameters | Physiologic/clinical significance |
|---|---|---|---|---|---|
| LEP | Instantaneous heat stimulation using CO2 or Thulium:YAG laser | A╬┤ fibers | N2ŌĆōP2 complex | Latency prolongation, amplitude reduction | Amplitude increases linearly with stimulus intensity [23]; reflects hyperexcitability and delayed conduction recovery [18,19,25]. |
| CHEP | Contact-type thermal stimulation using thermode | Mixed C+A╬┤ fibers | N2ŌĆōP2 complex | Amplitude change, age dependency | Shows longer latency and smaller amplitude than LEP; age-related attenuation indicates small-fiber dysfunction [24,25]. |
| SEP | Electrical stimulation of peripheral nerves (e.g., median nerve) | A╬▓ fibers | N20ŌĆōP30 | Latency, amplitude | Evaluates conduction velocity of large sensory fibers; used as a reference for comparing other EP modalities [21,22]. |
| AEP/VEP | Acoustic or visual stimulation | Sensory cortical pathways | N1ŌĆōP2, P100 | Latency | Reflects integrative responses across sensory cortices; employed for cross-modal EP comparisons [18,25]. |
Summary of major EP modalities used to assess sensory hyperexcitability. LEP and CHEP primarily reflect A╬┤- and C-fiber activity, respectively, and demonstrate prolonged latency and reduced amplitude under hyperexcitable or demyelinating conditions. SEP serves as a reference for large-fiber conduction, whereas AEP and VEP provide complementary information about multimodal cortical processing. Together, these EP paradigms allow objective, noninvasive evaluation of sensory conduction integrity and cortical synchrony in chronic itch and related neuropathic conditions.
EP, evoked potential; LEP, laser-evoked potential; CHEP, contact heat-evoked potential; SEP, somatosensory evoked potential; AEP, auditory evoked potential; VEP, visual evoked potential.
Table┬Ā2.
| Disease | Modality (LEP/CHEP/SEP) | Sample size | Reference site/stimulus | N2ŌĆōP2 latency | N2ŌĆōP2 amplitude | Control group | Key findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Atopic dermatitis | CHEP/EEGŌĆōEP | Ōēł 20ŌĆō25 patients | Forearm/thermal stimulus | Ōåæ Prolonged | Ōåō Reduced | Yes | EEGŌĆōEP combined analysis revealed delayed somatosensory activation and cortical hypo-responsiveness reflecting central sensitization and neural hyperexcitability. | [33] |
| Chronic kidney disease-associated pruritus | LEP | Ōēł 15ŌĆō20 patients | Hand/electrical stimulus | Ōåæ Prolonged | Ōåō Reduced | Yes | Delayed sensory conduction and decreased cortical synchrony indicating C- and A╬┤-fiber dysfunction and central sensitization. | [33,35] |
| Psoriasis | CHEP | Ōēł 12ŌĆō15 patients | Leg/thermal stimulus | Ōåæ Slightly prolonged | Ōåō Reduced | No | Reduced cortical responsiveness and delayed conduction suggest altered sensory processing within somatosensory cortex. | [35] |
| Experimental itch model (electrical stimulation-induced) | EEGŌĆōEP | Variable (n Ōēł 10ŌĆō15) | Wrist/electrical stimulus | Ōåæ Mildly prolonged | Ōåō Reduced | Yes | Selective C-fiber activation with corresponding cortical potential changes, confirming coupled peripheral hypersensitivity and cortical abnormalities. | [36] |
Summary of major EP modalities used to assess sensory hyperexcitability. LEP and CHEP primarily reflect A╬┤- and C-fiber activity, respectively, and demonstrate prolonged latency and reduced amplitude under hyperexcitable or demyelinating conditions. SEP serves as a reference for large-fiber conduction, whereas auditory EP and visual EP provide complementary information about multimodal cortical processing. Together, these EP paradigms allow objective, noninvasive evaluation of sensory conduction integrity and cortical synchrony in chronic itch and related neuropathic conditions.
EP, evoked potential; LEP, laser-evoked potential; CHEP, contact heat-evoked potential; SEP, somatosensory evoked potential; EEG, electroencephalography.
Table┬Ā3.
| Parameter | Typical setting | Rationale/notes | Reference |
|---|---|---|---|
| Stimulus intensity | 1.5ŌĆō2├Śindividual pain threshold | Ensures consistent activation of A╬┤- and C-fibers without tissue damage | [20,21] |
| Baseline temperature | 32ŌĆō35 ┬░C | Maintains thermode stability and minimizes latency variation | [21,29] |
| ISI | 8ŌĆō10 sec | Prevents habituation and allows full cortical recovery between stimuli | [29,47] |
| Number of repetitions | 20ŌĆō30 stimuli | Provides adequate averaging for stable N2ŌĆōP2 complex extraction | [24,25] |
| Electrode montage | CzŌĆōFzŌĆōA2 (10ŌĆō20 system) | Optimizes vertex recording of N2ŌĆōP2 with minimal noise | [18,19,22] |
| Filter range | 0.1ŌĆō30 Hz | Removes baseline drift and high-frequency artifacts | [24,25] |
| Averaging window | 1,000 msec post-stimulus | Captures both early (A╬┤) and late (C-fiber) components | [24,25] |
This table summarizes the core conditions recommended by the International Federation of Clinical Neurophysiology and normative laser-evoked potential/contact heat-evoked potential studies for reproducible EP acquisition. Consistent control of stimulus intensity, inter-stimulus interval, and electrode montage enhances comparability across studies and minimizes variability in N2ŌĆōP2 latency and amplitude, improving the reproducibility of EP-based sensory monitoring in chronic itch and related neuropathic conditions.
EP, evoked potential; ISI, inter-stimulus interval.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 191 View
- 4 Download
- ORCID iDs
-
Hye Seul Kim

https://orcid.org/0009-0000-0488-4550Seung Hoon Woo

https://orcid.org/0000-0001-7560-1140 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print