Advancements in intraoperative neuromonitoring for recurrent laryngeal nerve preservation in parathyroidectomy
Article information
Abstract
Parathyroidectomy is a pivotal surgical intervention for managing hyperparathyroidism, necessitating meticulous precision to preserve adjacent neural structures, particularly the recurrent laryngeal nerve. Intraoperative neuromonitoring (IONM) has emerged as an essential tool in minimizing nerve injury during parathyroidectomy. This review critically evaluates current neuromonitoring strategies, assessing their effectiveness in enhancing nerve preservation. It explores various IONM modalities, including intermittent and continuous monitoring, and examines their integration into surgical protocols. Additionally, advancements in neurophysiological monitoring technologies are discussed, highlighting their impact on surgical outcomes and patient quality of life. Integrated case studies illustrate the practical applications and outcomes of IONM in parathyroidectomy. Furthermore, an analysis of future directions explores potential innovations and improvements in neuromonitoring techniques. By synthesizing existing literature, clinical practices, and empirical evidence, this manuscript underscores the indispensable role of IONM in optimizing parathyroidectomy procedures, aiming to enhance patient safety and postoperative outcomes.
Introduction
Parathyroidectomy stands as the definitive surgical treatment for primary and secondary hyperparathyroidism, conditions characterized by the overproduction of parathyroid hormone [1]. The surgical procedure involves the precise removal of one or more hyperfunctioning parathyroid glands. Given the delicate anatomical relationships between the parathyroid glands and surrounding neural structures, particularly the recurrent laryngeal nerve (RLN), surgical precision is paramount [2]. Injury to the RLN can result in vocal cord paralysis, leading to voice hoarseness, swallowing difficulties, and, in severe cases, airway compromise [3]. These complications not only adversely affect patient quality of life but also necessitate additional medical interventions, thereby increasing healthcare costs and patient morbidity.
Intraoperative neuromonitoring (IONM) has revolutionized surgical practices by providing real-time feedback on the functional integrity of neural structures during surgery [4]. The integration of IONM into parathyroidectomy protocols represents a significant advancement in enhancing surgical precision and patient safety [5]. By facilitating the identification and preservation of the RLN and other critical nerves, IONM reduces the incidence of iatrogenic injuries, thereby improving postoperative outcomes [6,7].
This review aims to provide a comprehensive overview of neuromonitoring strategies in parathyroidectomy, highlighting their role in enhancing nerve preservation, evaluating their efficacy, and exploring future advancements in the field. Through an analysis of existing literature, recent case studies, and clinical practices, this manuscript underscores the indispensable role of IONM in optimizing parathyroidectomy procedures, ultimately aiming to improve patient safety and postoperative outcomes.
Neuromonitoring Techniques in Parathyroidectomy
Intraoperative neuromonitoring encompasses a variety of techniques designed to assess the functional status of nerves during surgical procedures [7]. In the context of parathyroidectomy, the primary focus is on monitoring the RLN to prevent inadvertent injury. Two main modalities are employed: intermittent neuromonitoring (INM) and continuous neuromonitoring (CNM), each offering distinct advantages and limitations [8].
Intermittent neuromonitoring involves periodic stimulation of the RLN at specific surgical milestones, typically before and after gland excision, to assess nerve integrity [9]. This approach is valued for its simplicity and ease of integration into standard surgical workflows. Surgeons visually identify the RLN and use nerve stimulators to intermittently verify its functionality [10]. Figure 1 illustrates the expected location of the RLN.
However, INM has limitations in detecting dynamic or transient nerve insults that may occur between monitoring points, potentially allowing unnoticed nerve stress or injury during critical surgical maneuvers [12]. Despite these limitations, INM remains widely adopted due to its cost-effectiveness and straightforward implementation [13]. For example, in a recent bilateral parathyroidectomy case, INM was utilized to assess RLN function before and after each gland excision, successfully preventing any nerve injury despite the surgery's complexity [14].
Continuous neuromonitoring, on the other hand, provides ongoing, real-time assessment of nerve function throughout the surgical procedure. Utilizing electromyography (EMG), CNM continuously records nerve signal transmission, allowing immediate detection of nerve stress or injury [4,15]. This modality offers a more sensitive approach to nerve preservation, enabling surgeons to respond promptly to any adverse changes in nerve function. Continuous monitoring is particularly beneficial in complex or prolonged surgeries where the risk of nerve injury is heightened [16]. The real-time feedback provided by CNM was instrumental in making immediate surgical adjustments, highlighting its critical role in enhancing surgical outcomes.
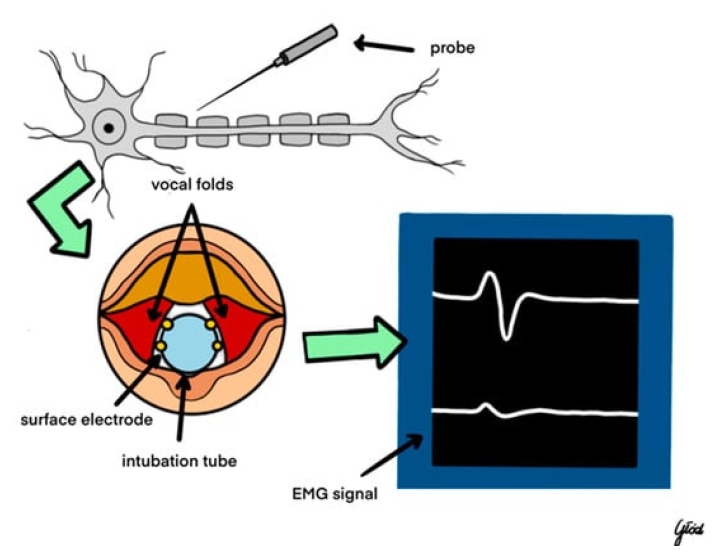
Electromyography serves as the cornerstone of IONM in parathyroidectomy, offering a quantitative measure of nerve function. By recording muscle re-sponses to nerve stimulation, EMG facilitates the detection of nerve irritation, stretching, or transection [17]. Key parameters monitored include the amplitude and latency of muscle responses, providing insights into nerve conduction integrity. Advanced EMG techniques, such as high-frequency stimulation and refined signal processing algorithms, have enhanced the precision and reliability of neuromonitoring, contributing to improved surgical outcomes [18,19].
Beyond EMG, other neuromonitoring modalities, such as somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP), are occasionally employed to provide additional layers of nerve function assessment [20]. However, their application in parathyroidectomy is less common compared to EMG, given the specific focus on motor function preservation of the RLN [21]. SSEP and MEP can offer supplementary information in complex cases involving multiple neural structures or when monitoring additional cranial nerves becomes necessary [4]. Nevertheless, the predominant reliance on EMG in parathyroidectomy underscores its suitability and effectiveness in addressing the specific neural challenges posed by this surgical procedure [22].
Protocols for Nerve Identification and Preservation
Effective neuromonitoring protocols are essential for maximizing the benefits of IONM in parathyroidectomy. These protocols encompass several key steps, beginning with thorough preoperative planning and extending through meticulous intraoperative nerve identification and continuous monitoring during gland excisions.
Preoperative planning involves comprehensive assessment and preparation before surgery. This includes utilizing high-resolution imaging techniques such as ultrasound and magnetic resonance imaging to accurately localize parathyroid glands relative to the RLN [23]. Such imaging facilitates targeted surgical approaches, thereby reducing the risk of inadvertent nerve injury. Additionally, preoperative assessment of vocal cord function, typically performed using laryngoscopy, establishes baseline data for postoperative comparison [24]. This baseline is crucial for detecting new nerve deficits and assessing RLN functional integrity after surgery.
During surgery, nerve identification is a critical phase. Surgeons first visually identify the RLN and then employ electrical stimulation coupled with EMG recording to confirm its location and functionality [25]. This dual approach enhances the accuracy of nerve localization, reducing the likelihood of accidental transection. For instance, in a robotic parathyroidectomy case, the integration of wireless IONM allowed seamless monitoring without impeding surgical access, ensuring efficient nerve preservation and improved surgical outcomes [8]. A visual representation of the IONM's operative system is showcased by Figure 2.
Monitoring during gland excisions involves maintaining vigilant oversight of nerve integrity. In inter-mittent monitoring, nerve stimulation occurs before and after gland removal to assess any changes in nerve function [26]. Continuous monitoring, conversely, provides real-time feedback on nerve activity throughout the excision process. This continuous feedback is invaluable for detecting nerve stress or injury as it occurs, allowing surgeons to make immediate adjustments to their technique [27]. In a recent case, continuous monitoring detected a subtle decrease in EMG amplitude during gland excision, prompting the surgical team to pause and reassess their approach, thereby preventing potential RLN transection [28].
Responding to neuromonitoring alerts requires immediate corrective actions to mitigate detected nerve stress or injury indicators. This may include pausing surgical manipulation, adjusting instrument positioning, or employing nerve protection strategies [29]. Documenting neuromonitoring data is also crucial for postoperative analysis and quality improvement, aiding in identifying patterns or recurrent issues that can be addressed through procedural refinements or targeted training [30]. For instance, in a reoperative parathyroidectomy case, neuromonitoring data revealed transient nerve stress, leading to modifications in surgical instruments and techniques, ultimately preserving nerve integrity and improving patient outcomes [31].
Postoperative evaluation involves analyzing neuromonitoring data and assessing nerve function after surgery. This includes reviewing EMG recordings and comparing pre- and postoperative vocal cord function to detect any new nerve deficits. Comprehensive patient counseling regarding neuromonitoring outcomes enhances patient understanding and satisfaction, as patients are informed about the measures taken to preserve nerve function and the implications of neuromonitoring results for their postoperative recovery [32].
Efficacy of Intraoperative Neuromonitoring in Nerve Preservation
The efficacy of IONM in reducing RLN injury rates during parathyroidectomy is well-documented through numerous studies and meta-analyses [33-35]. Some of the hallmark studies are listed in Table 1 for reference. These analyses consistently indicate that the use of IONM is associated with a statistically significant reduction in both transient and permanent RLN palsy compared to surgeries performed without neuromonitoring. This reduction is attributed to the real-time feedback provided by IONM, allowing immediate detection and correction of nerve insults during surgery.
Beyond statistical improvements in nerve preservation, IONM enhances surgical precision. By providing real-time feedback on nerve function, IONM enables surgeons to perform more accurate dissections and gland excisions, minimizing inadvertent nerve manipulation [41]. This improved precision translates into better postoperative functional outcomes, including the preservation of vocal cord movement and overall patient quality of life. Patients benefit from reduced incidences of voice hoarseness, swallowing difficulties, and other complications associated with RLN injury, leading to higher satisfaction rates and quicker recovery times [42,43].
Continuous EMG monitoring can detect subtle fluctuations in nerve activity during gland excision, prompting immediate surgical adjustments that preserved RLN function. Postoperative evaluations confirmed intact vocal cord mobility, underscoring the critical role of IONM in enhancing surgical outcomes. Furthermore, the use of IONM is associated with increased surgeon confidence, particularly in complex or reoperative cases where scar tissue and altered anatomy elevate the risk of nerve injury. Surgeons utilizing IONM report greater assurance in their ability to identify and preserve the RLN, contributing to more consistent surgical outcomes. This confidence not only improves the immediate surgical experience but also fosters a culture of safety and precision within surgical teams, promoting the adoption of best practices across institutions.
Technological Advancements in Neuromonitoring
The field of neuromonitoring has witnessed significant technological advancements, enhancing the precision and reliability of IONM in parathyroidectomy. These innovations address limitations of traditional monitoring techniques, offering surgeons more sophisticated tools to preserve nerve function during surgery.
One notable advancement is the development of wireless monitoring systems. By eliminating the need for wired connections, these systems offer increased surgeon mobility and reduced equipment clutter in the operative field. Enhanced mobility facilitates a more streamlined surgical workflow, allowing surgeons to maneuver more freely without being tethered to monitoring equipment. Advances in wireless technology have ensured signal fidelity and minimized interference from other electronic devices, maintaining the accuracy of nerve monitoring despite the removal of physical connections. The adoption of wireless systems has been particularly beneficial in minimally invasive and robotic-assisted surgeries, where space constraints make traditional wired setups cumbersome.
Miniaturized sensors represent another frontier in neuromonitoring technology. These sensors are designed to be more sensitive and durable, enhancing the accuracy of nerve monitoring, particularly in minimally invasive and robotic-assisted surgical approaches. Miniaturized sensors are compatible with confined surgical spaces, allowing precise monitoring without impeding surgical access. Additionally, the development of biocompatible materials minimizes tissue irritation, enhancing patient comfort and reducing the risk of postoperative complications. Innovations such as flexible and adhesive sensors conforming to nerve pathways are currently under development, promising enhanced integration with surgical techniques.
Advancements in signal processing and data visualization have also significantly improved the interpretability of neuromonitoring data. Enhanced algorithms for filtering and analyzing EMG signals reduce the incidence of false positives and negatives, providing more reliable feedback to surgeons. Improved data visualization tools present neuromonitoring information in intuitive formats, facilitating quicker and more accurate decision-making during surgery. These technological enhancements collectively contribute to the overall effectiveness of IONM, making it an indispensable component of modern parathyroidectomy procedures.
Outcomes and Clinical Implications
The integration of IONM into parathyroidectomy has yielded significant improvements in surgical outcomes and patient quality of life. The primary benefit observed is the reduction in nerve injury rates, consistently supported by clinical studies and metaanalyses. The use of IONM is associated with lower incidences of both transient and permanent RLN palsy, translating into fewer postoperative complications related to voice and swallowing functions. By providing real-time feedback on nerve integrity, IONM enables surgeons to avoid inadvertent nerve manipulation, thereby preserving nerve function and preventing the debilitating consequences of RLN injury.
Enhanced surgical precision is another key outcome associated with IONM use. Accurately identifying and monitoring the RLN during surgery allows for more precise gland excisions, minimizing the risk of nerve injury. This precision reduces the likelihood of postoperative complications and contributes to more favorable functional outcomes. Patients benefit from the preservation of vocal cord movement, maintaining normal voice quality and swallowing ability, thereby enhancing overall quality of life and reducing the need for additional medical interventions.
The cumulative effect of these outcomes positions IONM as a critical component of modern parathyroidectomy, shaping care standards and influencing future surgical practices in endocrine surgery. As IONM technologies and methodologies continue to evolve, their integration into parathyroidectomy protocols will likely become even more refined, further enhancing surgical precision and patient safety. The consistent improvement in clinical outcomes and patient satisfaction rates advocates for the broader adoption of IONM, establishing it as a standard of care in endocrine surgical procedures.
Challenges and Limitations
Despite the clear benefits of IONM in enhancing nerve preservation during parathyroidectomy, several challenges and limitations hinder its widespread adoption and optimal utilization. Addressing these challenges is essential for maximizing IONM effectiveness and ensuring accessibility across diverse surgical settings. Cost and resource allocation represent primary challenges associated with IONM implementation. The initial investment in neuromonitoring equipment can be substantial, particularly for smaller surgical centers or practices with limited financial resources.
Several studies have reported that IONM does not significantly reduce the incidence of RLN injuries during parathyroidectomy. For example, Pisanu et al. conducted a systematic review and meta-analysis comparing IONM with traditional visual nerve identification and found no statistically significant difference in RLN palsy rates between the two approaches [44]. Similarly, another study reported comparable outcomes in RLN preservation between IONM-assisted surgeries and those relying solely on anatomical visualization, suggesting that the theoretical benefits of IONM may be overstated [45]. Additional concerns have been raised regarding technical issues, such as signal interference and variability in neuromonitoring protocols, that could contribute to false positives or negatives, further complicating the interpretation of IONM data [46].
This conflicting evidence represents a notable limitation for both this review and the underlying methodological approach used to assess IONM efficacy. The heterogeneity in study designs, variable IONM protocols, and differences in surgeon expertise across the literature make it challenging to draw definitive conclusions about the protective benefits of IONM against RLN injury. Consequently, while IONM remains a promising adjunct in parathyroidectomy, the mixed findings underscore the need for further standardized, high-quality studies to more accurately delineate its role in nerve preservation and to guide its optimal implementation in clinical practice.
Conclusion
IONM has long been recognized as a valuable asset in parathyroidectomy, providing real-time feedback on RLN function and thereby enhancing surgical precision. Early investigations demonstrated that, when used alongside direct visualization, IONM can contribute to lower incidences of both transient and permanent RLN palsy [4,5]. However, contrasting evidence from other studies indicate that the benefits of IONM may not be as pronounced in all clinical settings, suggesting that its efficacy is influenced by factors beyond mere technological capability.
The variability in reported outcomes can largely be attributed to inconsistencies in neuromonitoring protocols, subjective interpretation of EMG signals, and technical challenges like signal interference. Such heterogeneity underscores the importance of establishing standardized guidelines and comprehensive training programs to ensure uniform application of IONM across diverse surgical environments. Without these measures, the potential of IONM may be undermined by operator-dependent factors and inconsistent methodological approaches, ultimately affecting patient outcomes.
Recent technological advancements are poised to address many of these limitations. Innovations such as wireless monitoring systems and miniaturized sensors have been shown to improve signal fidelity and reduce operator variability. These emerging tools promise to streamline the integration of IONM into the surgical workflow, making the technology more accessible and reliable even in high-risk or complex cases. However, while these developments are promising, their true clinical impact will depend on rigorous validation through prospective studies and real-world cost-benefit analyses.
In conclusion, the practical application of IONM in parathyroidectomy should be viewed as a complementary strategy that enhances, rather than replaces, meticulous surgical technique. Clinicians are encouraged to adopt IONM, particularly in complex or high-risk cases, while also investing in standardized protocols and advanced training to mitigate its inherent limitations. By harmonizing technological innovation with surgical expertise, IONM can be more effectively leveraged to improve patient safety and outcomes, paving the way for its broader, evidence-based adoption in endocrine surgery.
Notes
Funding
None.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
None.
Author Contributions
All work was done by KW.