Near-infrared spectroscopy for monitoring cerebral oxygenation in neurosurgical and critical care settings
Article information
Abstract
Near-infrared spectroscopy (NIRS) has emerged as a valuable non-invasive tool for monitoring cerebral oxygenation in neurosurgical and critical care settings. Maintaining adequate cerebral oxygenation is crucial to prevent irreversible brain injury and mortality. Traditional methods like intracranial pressure and cerebral perfusion pressure monitoring do not directly reflect cerebral blood flow or oxygen saturation. Invasive techniques such as jugular venous oxygen saturation and brain tissue partial pressure of oxygen monitoring have limitations in reflecting regional changes and carry inherent risks. This manuscript explores the application of NIRS technology for continuous and non-invasive assessment of cerebral oxygenation. NIRS utilizes near-infrared light to penetrate the skull and measure changes in oxygenated and deoxygenated hemoglobin concentrations in the brain tissue. This allows for real-time monitoring of cerebral oxygen saturation, providing valuable insights into the balance between oxygen supply and demand. The benefits of NIRS include its non-invasiveness, portability, continuous monitoring capability, and potential for detecting regional cerebral ischemia. This review discusses the principles of NIRS, its application in various neurosurgical procedures and critical care scenarios, and its potential to improve patient outcomes by enabling timely interventions to maintain optimal cerebral oxygenation.
Introduction
Maintaining adequate cerebral oxygenation is paramount in neurological settings to prevent the devastating consequences of irreversible brain injury and mortality [1]. When the brain's supply of blood and oxygen is completely interrupted, consciousness is lost within seconds, and prolonged anoxic conditions lead to irreversible damage and, in severe cases, brain death [2]. Therefore, effective methods for monitoring cerebral blood oxygen saturation during surgical procedures and the postoperative period are essential to protect cerebral function and improve patient outcomes [3]. Traditional methods for assessing brain health, such as intracranial pressure (ICP) and cerebral perfusion pressure (CPP) monitoring, while valuable, are limited in their ability to directly reflect cerebral blood flow or oxygen saturation [4]. Jugular venous oxygen saturation monitoring, an invasive technique involving the placement of a catheter in the jugular bulb, provides a global assessment of brain oxygenation and may not be sensitive enough to detect regional cerebral ischemia [5]. Similarly, brain tissue partial pressure of oxygen monitoring, which requires the implantation of microelectrodes into brain tissue, is invasive and may only reflect local oxygen levels, potentially misrepresenting the overall cerebral oxygen metabolism. These limitations of traditional invasive monitoring techniques underscore the need for non-invasive methods that can provide continuous and localized assessments of cerebral oxygenation.
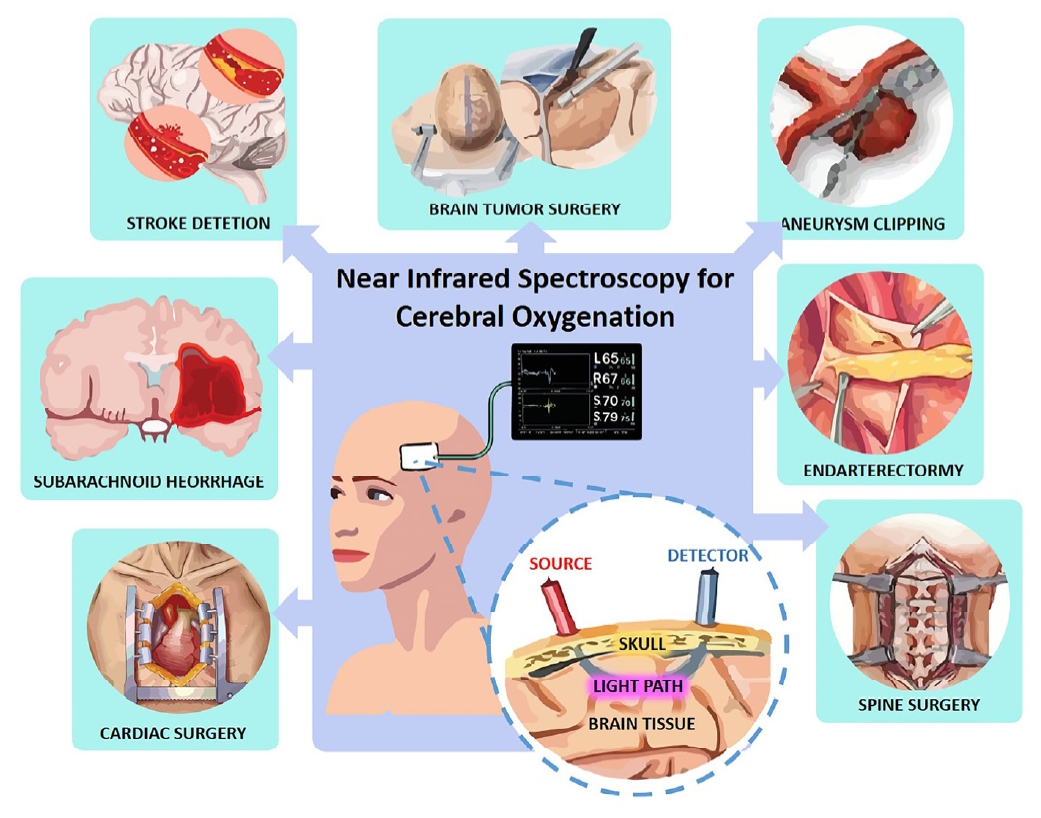
Near-infrared spectroscopy (NIRS) has emerged as a promising non-invasive neuromonitoring tool with the potential to overcome many of these limitations [6]. NIRS is a safe and readily available technology that can be used at the patient's bedside to provide continuous and repeatable data, facilitating the early detection of neurological deterioration [7]. Its non-invasive nature eliminates the risks associated with invasive procedures, making it suitable for a wide range of clinical scenarios, including monitoring non-brain injured patients who are at risk of secondary brain injury [8]. Furthermore, NIRS is considered a lowcost tool with significant potential for both diagnosis and treatment of patients who are at risk of neurological complications [9]. It allows for the online monitoring of tissue oxygenation in various clinical settings and can be utilized to assess cerebral perfusion and optimize cerebral blood flow [10]. This review aims to comprehensively examine the applications of NIRS in both neurosurgical and critical care settings, with a specific focus on its role in the detection of cerebral ischemia and its potential to guide clinical interventions. Figure 1 summarizes the clinical applications of NIRS. Having established the critical need for monitoring cerebral oxygenation to prevent irreversible brain injury and the emergence of NIRS as a valuable, non-invasive tool for this purpose, providing insights into cerebral oxygen saturation (ScO2), the following section will explore the underlying principles of this technology that enable such monitoring. Understanding these principles is crucial for appreciating how NIRS works and its capabilities in assessing cerebral oxygenation.
Principles of Near-Infrared Spectroscopy
The fundamental principle of NIRS relies on the interaction of near-infrared light with biological tissues [11]. Near-infrared light, typically in the wavelength range of 700 to 1100 nanometers, exhibits the property of penetrating tissue several centimeters due to relatively low absorption and dominant scattering by tissue components [12]. Within this nearinfrared window, key chromophores present in the brain, namely oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (HHb), and cytochrome c oxidase (CCO), exhibit distinct absorption spectra. This unique spectral signature allows NIRS to differentiate and quantify changes in the concentration of these chromophores, providing valuable information about tissue oxygenation and metabolic state. While light scattering in tissue complicates the direct measurement of absorption, it is also this scattering that enables the near-infrared light to travel through several centimeters of tissue, making non-invasive assessment of deeper tissues like the brain possible [13]. Broadband NIRS systems further exploit this principle by measuring changes in light attenuation across a wide range of wavelengths, enabling the retrieval of information not only about hemoglobin oxygenation but also about the redox state of CCO, which serves as an indicator of cellular energy utilization [14].
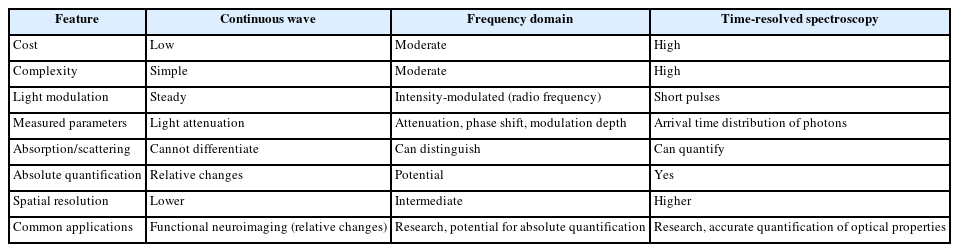
NIRS measurements are typically performed using one of three main spectroscopic techniques: Continuous wave (CW) spectroscopy, frequency domain (FD) spectroscopy, and time-resolved spectroscopy (TRS), also known as time domain (TD) spectroscopy [15]. CW spectroscopy represents the simplest and most widely used technique, involving the steady illumination of tissue with light and the detection of the transmitted light intensity [16]. While CW spectroscopy cannot directly differentiate between the effects of absorption and scattering, it allows for the reliable assessment of relative changes in blood volume and oxygenation, making it particularly useful for functional neuroimaging applications where changes over time are of primary interest. CW systems are generally cost-effective but rely on the assumption of homogenous tissue and a fixed path length of light through the tissue [17]. FD spectroscopy represents a more advanced technique where the light source is intensity-modulated at a high frequency (typically in the radio frequency range) [18]. By measuring both the attenuation of light intensity and the phase shift of the modulated signal as it passes through the tissue, FD spectroscopy allows for the distinction between absorption and scattering properties of the tissue and holds the potential for absolute quantification of chromophore concentrations [19]. However, FD systems are more complex and costly than CW systems and may exhibit a greater degree of noise in the recovered parameters [20]. TRS or TD spectroscopy utilizes extremely short pulses of light (on the order of picoseconds) and measures the distribution of arrival times of photons that have traveled through the tissue [21]. This technique provides the richest information about the optical properties of the tissue, enabling the quantification of both absorption and scattering coefficients with improved spatial resolution [22]. Despite its advantages, TD spectroscopy is often associated with cumbersome and expensive instrumentation, limiting its widespread clinical adoption. Comparison of NIRS measurement principles are listed in Table 1.
From these NIRS measurements, several key parameters can be derived, providing insights into cerebral oxygenation and hemodynamics [23]. ScO2, also referred to as regional cerebral oxygen saturation (rSO2), tissue oxygenation index (TOI) is a primary parameter that reflects the overall oxygen saturation within the cerebral tissue, encompassing a mixture of arterial, venous, and capillary blood. This parameter indicates the balance between oxygen delivery to the brain and oxygen consumption by the brain tissue [24]. It is important to note that different NIRS device manufacturers may use different terms and proprietary algorithms to calculate this value. Cerebral blood volume (CBV), which represents the total volume of blood in the cerebral tissue, can also be derived from NIRS measurements, often reflecting changes in the total hemoglobin concentration [25]. More advanced time-resolved NIRS techniques can even provide absolute measurements of CBV [25]. Furthermore, NIRS allows for the monitoring of changes in the concentrations of HbO2 and HHb based on their differential absorption of light at specific wavelengths [26]. Another derived parameter, fractional tissue oxygen extraction (FTOE), can be estimated by combining cerebral oxygenation measurements with peripheral oxygen saturation. FTOE provides an indication of the proportion of delivered oxygen that is extracted by the brain tissue, offering a surrogate marker of cerebral metabolism [27].
While NIRS offers a non-invasive window into cerebral oxygenation, it is important to consider its limitations in terms of depth of penetration and spatial resolution [28]. For brain imaging applications, the typical penetration depth of NIRS is approximately 3 centimeters [29], although this can vary depending on the wavelength of light used [30]. This depth limitation means that NIRS primarily allows for the assessment of the superficial cortical regions of the brain [29]. The spatial resolution achievable with NIRS is generally in the range of 5 to 10 millimeters, although some studies have reported resolutions of 2 to 3 centimeters [31]. More advanced techniques like time-domain near-infrared optical tomography have demonstrated the potential to achieve resolutions up to a depth of 30 millimeters in controlled liquid phantoms [32]. The sensitivity of NIRS to localized changes in light absorption can also vary depending on the specific monitoring device used [3]. However, advancements in high-density wearable NIRS systems are showing promise in achieving spatial resolutions comparable to functional magnetic resonance imaging (MRI). These considerations regarding penetration depth and spatial resolution are crucial for understanding the anatomical scope of NIRS measurements and for interpreting the resulting data in various clinical contexts. Having explored the fundamental principles of NIRS and how this non-invasive technology utilizes near-infrared light to measure key parameters like ScO2, also known as rSO2 or TOI, providing valuable insights into brain tissue oxygenation and metabolism, the succeeding section now delve into the specific clinical contexts where NIRS is applied.
NIRS in Neurosurgical Settings
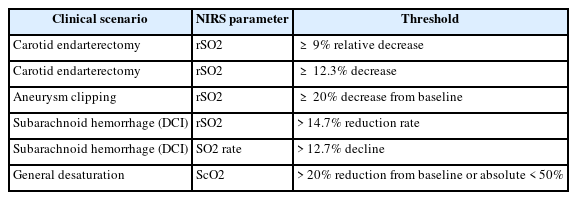
NIRS has found increasing applications in various neurosurgical settings, both during and after surgical procedures, to monitor cerebral oxygenation and detect potential ischemic events. Established and emerging thresholds for NIRS parameters indicative of is-chemia are listed in Table 2.
Intraoperative Neuromonitoring
During neurosurgical procedures, maintaining adequate cerebral oxygenation is critical to prevent intraoperative ischemic injury. NIRS has emerged as a valuable tool for intraoperative neuromonitoring in several specific neurosurgical contexts.
Carotid Endarterectomy
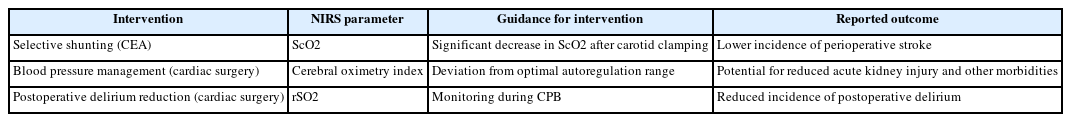
Carotid endarterectomy, a surgical procedure to remove plaque from the carotid artery, carries a risk of cerebral ischemia during the period of carotid artery clamping. Numerous studies have investigated the use of NIRS to detect cerebral ischemia in real-time during this critical phase of the surgery. NIRS allows for the continuous and non-invasive monitoring of cerebral oxygenation in patients undergoing carotid endarterectomy (CEA) [33] and has been shown to reliably identify the different stages of the procedure when performed under general anesthesia [34]. Its role in identifying patients who are at an elevated risk of perioperative stroke has also been highlighted. Research indicates that NIRS demonstrates high sensitivity and acceptable specificity in predicting cerebral ischemia and the subsequent need for intraoperative shunting [35]. For instance, a relative decrease in rSO2 of 9% has been associated with high sensitivity and specificity for predicting ischemic symptoms during carotid clamping [36]. Another study identified a 12.3% decrease in rSO2 as an optimal threshold for indicating intraoperative hypoperfusion as detected by transcranial doppler ultrasonography. It has also been observed that different thresholds for rSO2 decrease may be indicative of neurological distress in patients with symptomatic versus asymptomatic carotid stenosis [37]. Furthermore, NIRS monitoring, specifically the percentage change in rSO2 (NIRS%), has shown comparable accuracy to stump pressure measurement and superior accuracy to somatosensory evoked potentials in detecting cerebral ischemia during CEA [38]. Based on these findings, NIRS has been utilized to guide selective shunting strategies during CEA, where shunt placement is contingent on significant changes in ScO2 [39]. Studies have demonstrated that a NIRS-guided selective shunting approach is associated with a lower incidence of perioperative stroke compared to a strategy of routine shunting in all patients [40] and has the potential to identify patients at risk of hypoperfusion during carotid clamping, leading to excellent clinical outcomes.
Aneurysm Clipping and Coiling
The surgical clipping or endovascular coiling of intracranial aneurysms often involves temporary occlusion of blood vessels, which can pose a risk of cerebral ischemia. NIRS has been employed to monitor cerebral oxygenation during these critical periods [41]. In patients with poor-grade aneurysmal subarachnoid hemorrhage (aSAH) undergoing coiling, NIRS offers a simple, continuous, real-time, and non-invasive method for cerebral monitoring [13]. It can be used to determine cerebral tissue oxygenation and monitor for ischemic events during endovascular procedures [42], allowing for the early detection of such events and the potential for timely intervention [43]. During the surgical clipping of giant cerebral aneurysms, a study found that a 20% decrease in rSO2 from the preclamp baseline was a predictor of neurological compromise, exhibiting a sensitivity of 80% and a specificity of 82.2% [44]. NIRS also shows promise as a tool for the real-time detection of delayed cerebral ischemia (DCI) in patients with poorgrade SAH at the bedside [45].
Brain Tumor Surgery
During the resection of brain tumors, there is a risk of vascular compromise that can lead to cerebral ischemia. NIRS monitoring has been utilized in this setting to detect such events [46]. NIRS is increasingly employed for cerebral oxygenation monitoring in neurosurgical procedures, including brain tumor surgeries [47]. It allows for the assessment of cerebral perfusion in the vicinity of the tumor, providing continuous and measurable information about cerebral oxygenation throughout the surgery [41].
Spine Surgery
Patients undergoing spine surgery, particularly in the prone position, may be at risk of cerebral hypoperfusion due to altered hemodynamics. Cerebral NIRS monitoring has been investigated for its potential to detect such hypoperfusion in these cases [13]. While some studies have not found significant differences in cerebral oxygenation between the supine and prone positions, even with observed drops in blood pressure and heart rate [42], others have reported a slow reduction in cerebral oxygenation in both sitting and prone positions during posterior fossa tumor surgery. One study found a significant decrease in cerebral oxygenation during the mid and end stages of spinal surgery in the prone position, noting a positive correlation between mean arterial pressure and cerebral oxygenation [48]. Given these potential risks, the use of NIRS monitoring is recommended for patients with comorbidities and those undergoing prolonged spine surgeries in the prone position [47].
Pediatric Neurosurgery
Monitoring cerebral oxygenation in children undergoing neurosurgical procedures presents unique considerations. NIRS has shown to be of added value in neonatal intensive care for this purpose, as systemic arterial oxygenation may not always reflect cerebral oxygenation accurately [49]. It offers a non-invasive method to monitor global brain oxygenation in neonates and can be used in conjunction with amplitude-integrated electroencephalography (aEEG) [49]. However, a cochrane review assessing the effects of perioperative cerebral NIRS monitoring in both children and adults found uncertain evidence regarding its impact on neurological injury, stroke, delirium, and death, with little to no decrease in postoperative cognitive decline [50]. Conversely, a case report highlighted the utility of NIRS in the early detection and timely intervention to prevent prolonged cerebral ischemia in a 2-year-old child undergoing surgery for a vascular ring [51]. A systematic review evaluating the effects of clinical care with access to cerebral NIRS monitoring in children and adults concluded that the evidence for its benefits on mortality and neurological deficit is very uncertain [52].
Post Operative Neuromonitoring
NIRS plays an important role in the postoperative period, particularly in the neurosurgical intensive care unit (ICU), for the continuous monitoring of cerebral oxygenation and the early detection of complications. Continuous NIRS monitoring is feasible and valuable in the neurosurgical ICU for critically ill patients [53]. In patients who have experienced a SAH, NIRS can aid in the early detection of DCI [54]. Studies have shown significant differences in rSO2 levels between patients who develop DCI and those who do not, particularly between days 7 and 9 post-hemorrhage [40]. A decline of more than 12.7% in the rSO2 rate has demonstrated high sensitivity and specificity for identifying DCI, indicating that NIRS is a feasible method for the real-time detection of this serious complication [55]. NIRS is also utilized for monitoring cerebral oxygenation in patients with traumatic brain injury (TBI) in the postoperative period [41]. It has long been recognized as a promising non-invasive tool for monitoring cerebral tissue oxygenation and perfusion in the context of TBI [13] and has the potential for earlier initiation of monitoring with minimal interoperator variability [45]. NIRS can detect intracranial bleeding and assess brain tissue oxygenation and cerebral perfusion in TBI patients [43]. Research suggests that higher rSO2 levels are associated with survival in TBI patients, whereas values below 68% may indicate a critical threshold for increased mortality risk [45].
NIRS in Critical Care Settings
Building on the previous discussions of NIRS in neurosurgery (including intra- and post-operative applications), this section will now explore its significant and expanding role in critical care settings such as TBI, Stroke, and SAH, where continuous non-invasive cerebral oxygenation monitoring is equally critical for detecting complications and informing clinical decisions.
Traumatic Brain Injury
In the critical care management of TBI, NIRS serves as a valuable tool for continuous monitoring of cerebral oxygenation and hemodynamics [45]. While some studies have explored the correlation between NIRS parameters and ICP as well as CPP [44], the sensitivity of NIRS in reliably detecting or predicting changes in ICP remains limited, although some temporal relationships have been observed [48]. NIRS can also be used to detect secondary insults such as ischemia and hypoxia in TBI patients [52], and it has shown sensitivity in detecting severe cerebral hypoxia (PbrO2 < 12 mm Hg) but may be less effective in milder cases [56]. Furthermore, NIRS plays a role in guiding management strategies aimed at optimizing cerebral oxygenation in TBI patients, with proposed algorithms for clinical decision-making based on cerebral desaturation episodes [57].
Stroke
NIRS has potential applications across the spectrum of stroke care. In the acute phase of ischemic stroke, it has been investigated for its ability to aid in the early detection of the penumbra and the monitoring of collateral circulation [57]. However, one study found that NIRS monitoring during endovascular treatment for acute ischemic stroke did not detect significant differences in rSO2 between hemispheres or changes within a hemisphere over time [50]. NIRS also has a role in monitoring cerebral oxygenation during and after thrombolysis or thrombectomy procedures [52]. In the management of hemorrhagic stroke, particularly subarachnoid hemorrhage, cerebral oximetry using NIRS has demonstrated utility in the diagnosis of secondary hypoperfusion and cerebral vasospasm [52].
Subaracnoid Hemorrhage
As previously mentioned, continuous NIRS monitoring is valuable for the detection of DCI in patients with SAH. Changes in NIRS parameters, such as rSO2, have been shown to correlate with the occurrence and severity of angiographic vasospasm, a major contributor to DCI [58]. This suggests that NIRS can potentially serve as a tool to guide prophylactic or therapeutic interventions aimed at preventing or treating DCI in this high-risk population, with proposed decision-making algorithms based on cerebral desaturation [59].
Neonatal and Pediatric Critical Care
Cerebral oxygenation monitoring is of paramount importance in neonates and children who are neurologically compromised. NIRS has found widespread applications in this population, particularly in conditions such as hypoxic-ischemic encephalopathy (HIE), congenital heart disease, and other critical illnesses [49]. In infants with HIE, NIRS can provide continuous bedside information about brain hemodynamics, oxygenation, and metabolism. It is also widely used in pediatric cardiac surgery to monitor tissue oxygenation [38]. Furthermore, NIRS is utilized to monitor the effects of various interventions designed to improve cerebral oxygenation in this vulnerable population [60]. Studies have indicated that specific NIRS-derived parameters, such as cerebral ScO2, can significantly predict adverse outcomes in infants with HIE within the first 24 to 72 hours of life in some instances [60].
Cardiac Surgery and Extracorporeal Circulation
NIRS monitoring plays a significant role in detecting cerebral hypoperfusion during cardiopulmonary bypass (CPB) in patients undergoing cardiac surgery [61]. While the correlation between intraoperative NIRS desaturations and postoperative neurological outcomes is still under investigation, NIRS-guided strategies are being explored to optimize cerebral perfusion during these complex procedures [62]. For instance, the determination of cerebral pressure-flow autoregulation limits is now possible using processed oximetry signals in relation to arterial pressure [42]. Notably, recent research suggests that NIRS monitoring during cardiac surgery with CPB can help reduce the incidence of postoperative delirium, a common complication in this patient population [63].
NIRS in Detecting Cerebral Ischemia and Guiding Interventions
Beyond the neurosurgical operating room and immediate postoperative period, NIRS has found significant utility in various critical care settings for monitoring cerebral oxygenation and guiding management. A summary of NIRS-guided interventions and outcomes are listed in Table 3.
Sensitivity and Specificity of NIRS for Ischemia Detection
The accuracy of NIRS in identifying cerebral ischemia has been evaluated in numerous studies, often compared against gold standard methods such as microdialysis and imaging techniques [64]. One study proposed a correlation between a novel NIRS sensor and a brain tissue oxygen probe in detecting ischemic events. However, the reliability of NIRS measurements can be influenced by several factors, in cluding extracranial contamination from superficial tissues and inherent physiological variability [65]. The rSO2 signal can be affected by anatomical confounders such as the thickness of extracranial tissues, the skull, and the cerebrospinal fluid, as well as dynamic factors like anemia, acid-base status, and changes in arteriovenous blood partitioning [66]. The presence of intermediate structures like skin, skull, meninges, and cerebrospinal fluid can significantly influence NIRS results [67]. Erroneous NIRS values may also arise due to altered tissue boundary conditions during surgery or inadequate penetration depth of the sensors [68]. Furthermore, the use of different NIRS devices and proprietary algorithms by various manufacturers can lead to non-comparable absolute oxygenation values, posing challenges for standardization and interpretation across different studies and clinical settings [67].
Threshold and Interpretation of NIRS Data
Establishing clear thresholds for significant changes in NIRS parameters that are indicative of cerebral ischemia is crucial for clinical decision-making. Several studies have proposed and investigated various thresholds for different neurological conditions [69]. For instance, in carotid endarterectomy, a 12.3% decrease in rSO2 has been identified as an optimal threshold for indicating intraoperative hypoperfusion [39], while different thresholds may be necessary for symptomatic and asymptomatic patients [1]. In the context of subarachnoid hemorrhage, a reduction in rSO2 greater than 14.7% has been associated with delayed cerebral ischemia [2], and a decline of more than 12.7% in the SO2 rate has shown high sensitivity and specificity for DCI detection in another study [14]. During aneurysm clipping, a 20% decrease in rSO2 from baseline predicted neurological compromise with good accuracy [22]. A widely used general paradigm for defining cerebral desaturation is a reduction of more than 20% from the patient's baseline value or an absolute cerebral oxygen saturation value of less than 50%. Given the inherent variability in baseline cerebral oxygen saturation values among individuals, monitoring trends in NIRS data is often considered more clinically useful than relying on isolated absolute measurements [38]. Integrating NIRS data with other physiological monitoring modalities, such EEG in neonates, can provide a more comprehensive assessment of the patient's neurological status and improve the interpretation of NIRS findings [41].
NIRS-Guided Interventions
Real-time NIRS monitoring has been successfully used to guide clinical decision-making in various neurosurgical and critical care scenarios [48]. In carotid endarterectomy, NIRS monitoring facilitates the implementation of selective shunting strategies, where a significant drop in cerebral oxygen saturation triggers the placement of an intraluminal shunt [50]. During cardiovascular surgery, NIRS can be used to determine the limits of cerebral pressure-flow autoregulation, allowing for the optimization of blood pressure management [58]. Furthermore, NIRS monitoring during cardiac surgery with cardiopulmonary bypass has been shown to help reduce the incidence of postoperative delirium [49]. Based on NIRS readings, clinicians can make adjustments to blood pressure, ventilation, and other physiological parameters to maintain adequate cerebral oxygenation [60]. For instance, in cases of cerebral desaturation detected by NIRS, interventions such as increasing mean arterial pressure, adjusting arterial partial pressure of oxygen, or modifying the fraction of inspired oxygen may be implemented [42]. There is growing evidence suggesting that NIRS-guided management can lead to improved neurological outcomes in certain clinical settings, such as the association of NIRS-guided selective shunting with a lower rate of perioperative stroke in patients undergoing carotid endarterectomy [65].
Limitations and Future Directions
Despite its numerous advantages, NIRS technology has several limitations that need to be considered in clinical practice and future research. One of the primary limitations is the potential for extracranial contamination, where the NIRS signal may be influenced by oxygenation changes in superficial tissues of the head rather than solely reflecting cerebral oxygenation. Variability in the optical properties of different tissues among individuals can also affect the accuracy of NIRS measurements. As previously discussed, the depth of penetration of near-infrared light is limited to approximately 3 centimeters in the brain, primarily allowing assessment of the superficial cortex, and the spatial resolution is generally in the range of 5 to 10 millimeters. Systemic physiological factors, such as anemia and changes in acid-base status, can also influence NIRS readings. Additionally, NIRS device variability complicates this in that different manufacturers may use different terms and proprietary algorithms to calculate cerebral oxygen saturation. For example, ScO2, also referred to as rSO2, TOI is a primary parameter, but different manufacturers may use different terms and proprietary algorithms for its calculation. This use of different devices and proprietary algorithms by various manufacturers can lead to non-comparable absolute oxygenation values, which poses challenges for standardization and interpretation across different studies and clinical settings. These variations make it difficult to compare data obtained from different devices. These makes standardization and interpretation of NIRS data across different devices and clinical settings highly challenging due to variations in algorithms and the potential measurement of different parameters (e.g., rSO2 versus TOI).
In specific patient populations, such as those with significantly high hematocrit, NIRS monitoring may even fail. Conditions like scalp injury, skull fracture, or recent craniotomy or cranioplasty can also compromise the accuracy of NIRS measurements. Future research and development in the field of NIRS are actively addressing these limitations and exploring new avenues for its application [36]. Advancements in NIRS technology are focusing on improving signal quality and depth penetration through techniques such as multi-distance NIRS, which is already used to measure cerebral tissue saturation [15], and diffuse correlation spectroscopy, which can provide more specific information about tissue perfusion and blood flow by measuring subtle fluctuations in light scatter [60]. The integration of NIRS with other neuromonitoring modalities, such as EEG and ICP monitoring, as well as with neuroimaging techniques like MRI through neuronavigation systems, holds promise for providing a more comprehensive understanding of brain function and pathology [50]. The development of sophisticated algorithms and the application of machine learning techniques are being explored for automated analysis and interpretation of NIRS data, potentially enabling real-time inference of biochemical composition changes in brain tissue. Researchers are also investigating new applications of NIRS in various other neurological conditions, including epilepsy and migraine, as well as in the study of neurodevelopmental disorders and the monitoring of cortical function in healthy adults and those at risk of dementia. Finally, large-scale clinical trials are essential to further validate the role of NIRS in improving patient outcomes across different neurosurgical and critical care settings.
Conclusion
The current evidence strongly supports the use of NIRS as a valuable tool for monitoring cerebral oxygenation in both neurosurgical and critical care settings. NIRS offers a non-invasive, continuous, and readily available method for assessing brain health in a variety of clinical scenarios. Its role in detecting cerebral ischemia has been demonstrated in procedures like carotid endarterectomy and in the management of conditions such as subarachnoid hemorrhage, where early detection of delayed cerebral ischemia is crucial. Furthermore, NIRS has shown potential in guiding clinical interventions, such as selective shunting during CEA and the optimization of cerebral perfusion in cardiac surgery. Despite its strengths, NIRS technology has limitations, including its limited depth of penetration and spatial resolution, susceptibility to extracranial contamination, and challenges in standardization across different devices. These limitations necessitate careful interpretation of NIRS data, often in conjunction with other physiological monitoring. Looking ahead, ongoing research and development efforts are focused on overcoming these limitations through technological advancements and the integration of NIRS with other neurodiagnostic tools. Large-scale clinical trials will be critical in further validating the clinical utility of NIRS and establishing its role in improving neurological patient care. As the technology continues to evolve, NIRS holds significant potential to become an even more integral component of neuromonitoring in the quest to improve outcomes for patients with neurological compromise.
Notes
Funding
None.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
None.
Author Contributions
All work was done by AP.