Introduction
Mechanisms of Taste Transduction
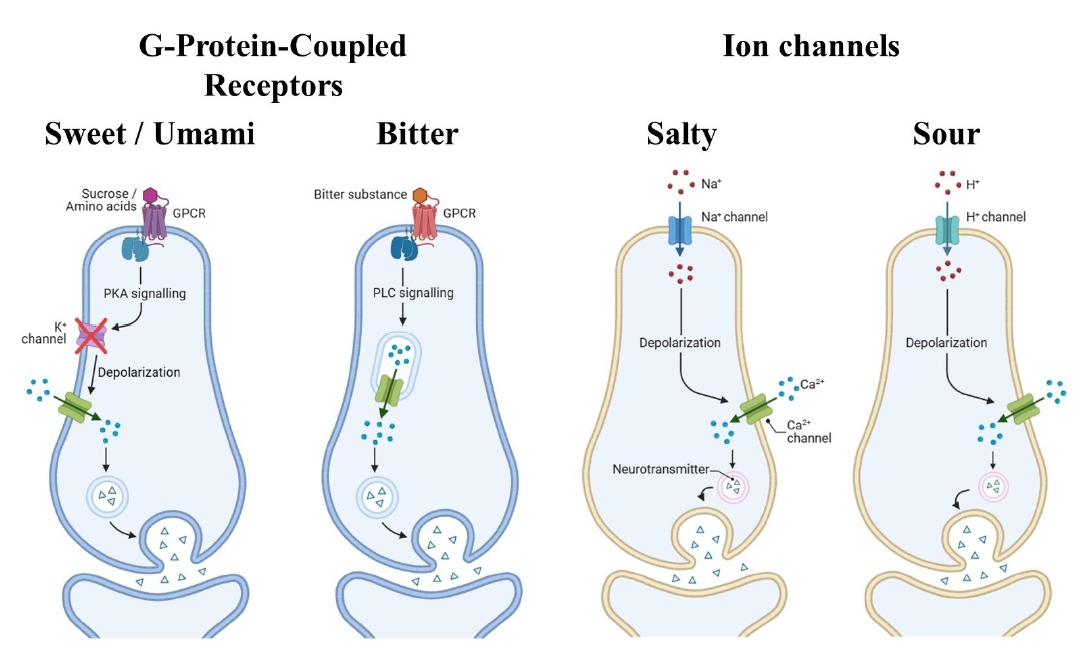
G-Protein-Coupled Receptors for Sweet, Umami, and Bitter Tastes
Ion Channels for Salty and Sour Tastes
Secondary Messengers and Intracellular Signaling Pathways
Integration of Taste Signals
Electrophysiology of Taste Cells
Electrophysiological Techniques in Taste Research
Patch-Clamp Recording
Electrochemical and Voltage-Sensitive Dye Techniques
Multi-Electrode Arrays
Findings in the Electrophysiology of Taste Cells
Voltage-Gated Ion Channels
Ligand-Gated Ion Channels
Neurotransmitter Release and Synaptic Activity
Temporal Dynamics of Taste Responses
Role of Electrophysiology in Decoding Taste Perception
Role of Electrophysiology in Decoding Taste Perception
Peripheral Encoding of Taste
Labeled-Line Model
Across-Fiber Pattern Model
Temporal Dynamics in Taste Buds
Central Processing of Taste Signals
Neurons in the Nucleus of the Solitary Tract
Cortical Representation of Taste
Electrophysiology and Taste Modulation
Neuromodulation of Gustatory Responses
Plasticity in Taste Perception
Decoding Taste at the Population Level
Advances in Electrophysiological Techniques in Taste Research
Modern Innovations in Electrophysiology
Optogenetics
Microfluidic Devices
Electrode Arrays
Integration of Electrophysiology with Other Techniques
Electrophysiology and Genomics
Neuroimaging and Electrophysiology
Future Directions
1. Development of In Vivo Recording Systems: Advancements in miniaturized and wireless electrophysiological devices will enable long-term, real-time recordings of taste responses in freely moving animals. These systems could provide insights into how taste perception is influenced by behavioral context and physiological states, such as hunger or satiety.
2. Single-Cell Electrophysiology in Organ-on-a-Chip Models: Combining electrophysiology with organ-on-a-chip technologies could allow for the study of human taste cells in controlled environments, bridging the gap between animal models and human physiology.
3. Artificial Intelligence and Machine Learning: The application of artificial intelligence and machine learning algorithms to analyze electrophysiological data could uncover novel patterns and relationships in taste coding. These tools could also aid in the development of predictive models for taste responses to novel tastants.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print