Management strategies for suspected nerve injury during thyroid surgery: a comprehensive review
Article information
Abstract
Thyroid surgery, such as thyroidectomy and lobectomy, is commonly performed to treat thyroid disorders. Nerve injury, especially to the recurrent laryngeal and superior laryngeal nerves, is a major complication, leading to vocal cord paralysis, dysphagia, and reduced quality of life. This review explores strategies for managing suspected nerve injury during thyroid surgery, including preventive measures, intraoperative neuromonitoring, surgical techniques for nerve preservation, and postoperative rehabilitation. Techniques like electromyography, electroglottography, and direct nerve stimulation help detect nerve damage early. Immediate responses, including microsurgical repair, are crucial. Postoperative care involves voice therapy, pharmacological treatment, and psychosocial support. Technological advancements, such as high-definition nerve imaging, robotic-assisted surgery, and artificial intelligence, improve nerve preservation. Effective management requires a multifaceted, multidisciplinary approach to minimize nerve injury and improve patient outcomes. Future research in regenerative medicine and personalized surgical planning holds promise for further advancements.
Introduction
Thyroid surgery, encompassing procedures such as thyroidectomy and lobectomy, is widely performed to address a spectrum of thyroid disorders, including benign nodules, hyperthyroidism, and thyroid malignancies [1]. Despite significant advancements in surgical techniques and perioperative care, nerve injury remains a formidable complication, with the recurrent laryngeal nerve (RLN) and the superior laryngeal nerve (SLN) being the most frequently affected [2]. These nerve injuries can lead to severe consequences such as vocal cord paralysis, dysphagia, aspiration pneumonia, and overall diminished quality of life, necessitating the development and implementation of effective management strategies [3].
The Pathophysiology of Nerve Injury
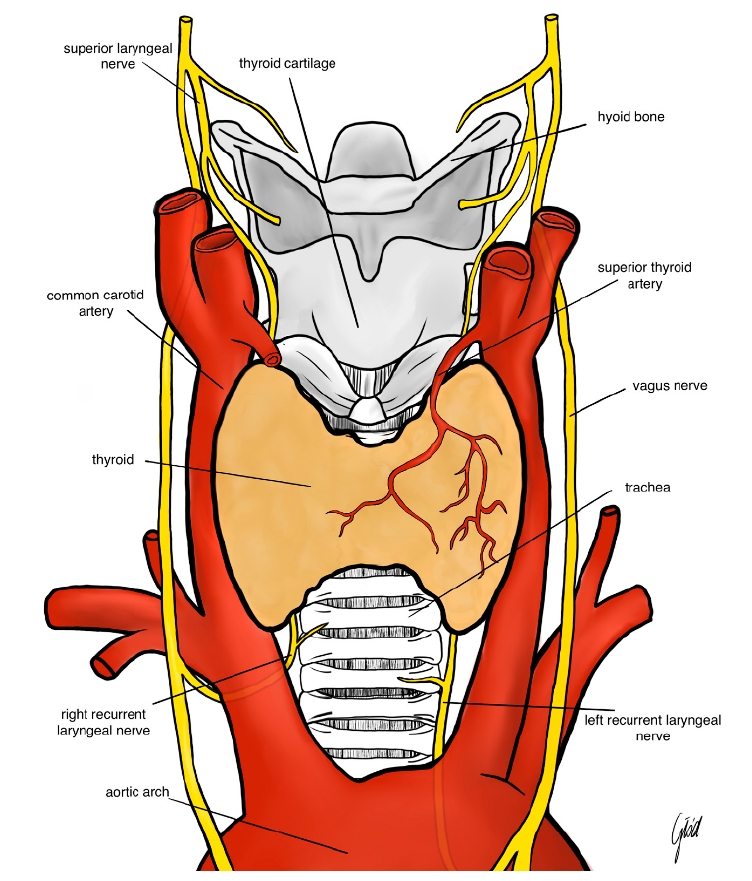
The pathophysiology of nerve injury in thyroid surgery is multifaceted. The RLN, which innervates the vocal cords, is particularly vulnerable due to its anatomical course, which often runs in close proximity to the thyroid gland [4]. Injury to the RLN can result from direct trauma by surgical instruments, excessive stretching from retraction, thermal injury from electrocautery, or ischemia due to compromised blood supply [5]. Similarly, the SLN, responsible for controlling pitch modulation and voice projection, can be inadvertently damaged during ligation of the superior thyroid artery [6]. The spectrum of nerve injury ranges from neuropraxia, characterized by temporary loss of nerve function without structural damage, to neuro-tmesis, which involves complete transection of the nerve fibers [7]. Understanding these mechanisms is crucial for both prevention and effective management of nerve injuries (Figure 1).
Intraoperative Neuromonitoring
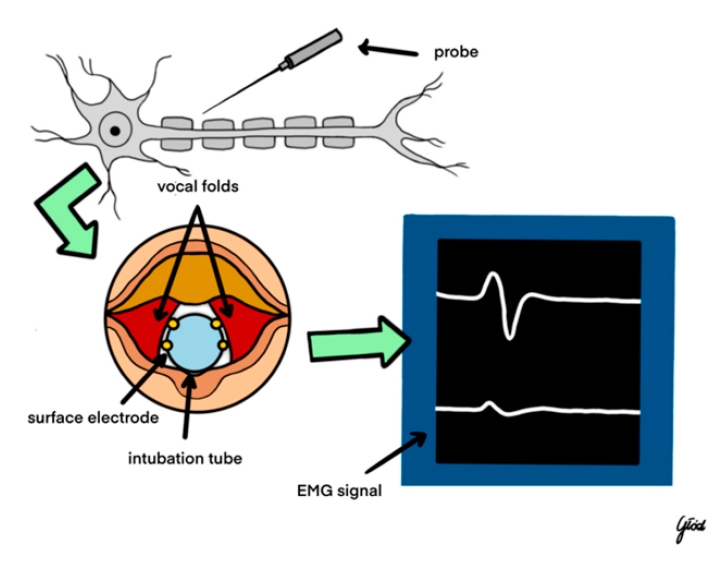
Intraoperative neuromonitoring (IONM) has emerged as a pivotal tool in the early detection and prevention of nerve injuries during thyroid surgery [8]. Techniques such as electromyography (EMG), electroglottography (EGG), and direct nerve stimulation are integral components of IONM. EMG provides real-time feedback on muscle electrical activity, allowing surgeons to detect abnormal nerve function indicative of impending or existing nerve damage [9]. EGG assesses vocal cord function by measuring glottal closure, thereby offering indirect evidence of RLN integrity [10]. Direct nerve stimulation involves the application of electrical impulses to identify and preserve nerve pathways, with continuous or intermittent stimulation serving as an immediate alert system for potential nerve compromise [11]. Studies have consistently demonstrated that the utilization of IONM significantly reduces the incidence of permanent RLN injuries compared to visual identification alone [12]. Furthermore, standardized IONM protocols enhance the reliability and consistency of nerve monitoring during thyroidectomy, contributing to improved surgical outcomes [13].
Upon suspicion of nerve injury during surgery, prompt and decisive surgical response is imperative to mitigate further damage and optimize recovery [14]. The initial step involves immediate visual inspection of the affected nerve to identify any visible trauma, such as transection or compression [15]. If nerve injury is confirmed, modifying surgical techniques becomes essential. This may include reducing retraction forces, repositioning instruments, or altering dissection planes to alleviate undue stress on the nerve [16]. In cases where the nerve is transected, microsurgical repair techniques, such as end-to-end anastomosis or nerve grafting, should be employed to restore nerve continuity and function [17]. Additionally, temporary nerve protection strategies, including the application of protective barriers or cushioning materials, can shield the nerve from further trauma during the remainder of the surgical procedure [18].
Postoperative Management of Nerve Injury
Postoperative management of nerve injury focuses on minimizing the functional impact and promoting nerve regeneration [19]. Voice therapy is a cornerstone of postoperative care, aiding patients in compensating for vocal cord paralysis and improving overall voice quality [20]. Speech and language therapists work with patients to develop strategies for effective communication and swallowing, thereby enhancing their quality of life [21]. Pharmacological interventions, such as anti-inflammatory medications and neuroprotective agents, may be administered to reduce nerve inflammation and support the regeneration process [22]. Regular postoperative monitoring, including laryngoscopy, is essential to assess vocal cord function and guide ongoing rehabilitation efforts [23]. Moreover, addressing the psychosocial aspects of nerve injury through counseling and support groups is crucial for comprehensive patient care, as voice changes can have significant emotional and psychological repercussions [24].
Preventive measures play a critical role in reducing the incidence of nerve injuries during thyroid surgery. Meticulous surgical planning and execution are paramount, beginning with a thorough understanding of thyroid gland anatomy and the anatomical variations of the RLN [25]. Surgeons must be vigilant in identifying nerve structures and avoiding excessive manipulation or stretching during dissection [26]. Nerve-sparing techniques, such as meticulous dissection and minimal use of energy devices near nerve sites, are essential to preserve nerve integrity [27]. Preoperative imaging modalities, including ultrasound and magnetic resonance imaging, can aid in identifying anatomical variations and guiding the surgical approach, thereby minimizing the risk of nerve injury [28]. Continuous education and training for surgical teams on advanced thyroidectomy techniques and the latest developments in nerve preservation are also vital components of effective preventive strategies [29].
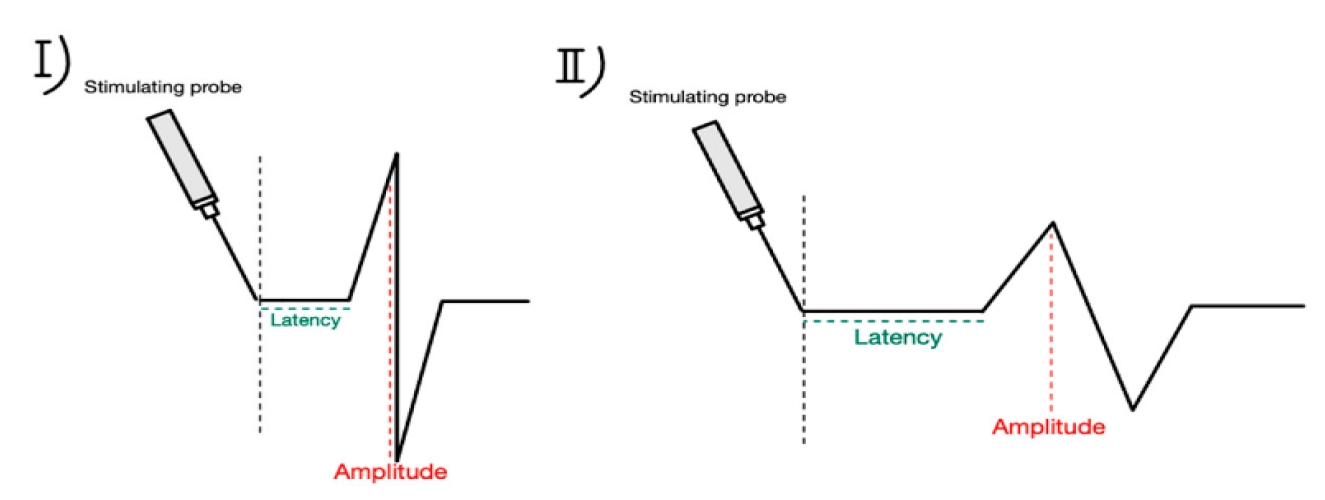
Technological advancements have significantly enhanced nerve preservation during thyroid surgery. High-definition nerve imaging, utilizing intraoperative ultrasound and nerve visualization systems, provides surgeons with enhanced visualization of nerve structures, facilitating precise dissection and minimizing inadvertent damage [30]. Robotic-assisted surgery offers superior dexterity and magnification, enabling meticulous identification and preservation of nerve pathways [31]. The integration of artificial intelligence into IONM systems has further improved the accuracy and predictive capabilities for nerve injury, allowing for real-time data analysis and more informed surgical decision-making [32] (Figure 3).
Augmented reality systems, which overlay anatomical information onto the surgical field, provide surgeons with enhanced spatial awareness and nerve localization, thereby reducing the risk of inadvertent nerve damage [33]. These technological innovations collectively contribute to improved surgical precision and patient outcomes [34].
A multidisciplinary approach is essential for the effective management of nerve injuries in thyroid surgery. Seamless coordination and communication between surgeons, anesthesiologists, neurophysiologists, and rehabilitation specialists ensure comprehensive care throughout the surgical continuum [35]. Ongoing training and education for surgical teams on the latest nerve-sparing techniques and advancements in IONM are crucial for maintaining high standards of surgical practice [36]. Simulation training, utilizing surgical simulators to practice nerve preservation and injury management, enhances surgical skills and preparedness, thereby reducing the likelihood of nerve injury [37]. Additionally, patient education about the risks of nerve injury, potential outcomes, and the importance of postoperative rehabilitation fosters informed decision-making and compliance with rehabilitation protocols, ultimately contributing to better recovery and quality of life [38].
Clinical evidence underscores the effectiveness of comprehensive management strategies in reducing the incidence and impact of nerve injuries during thyroid surgery. For instance, a study by Thompson et al. demonstrated that the implementation of advanced IONM techniques reduced the incidence of permanent RLN injuries by 25% in thyroidectomy procedures [39]. A case report by Martinez highlighted the successful recovery of an RLN injury through immediate microsurgical intervention and intensive voice therapy, emphasizing the importance of prompt and appropriate postoperative care [40]. Additionally, a systematic review by Lee et al. confirmed that multidisciplinary management approaches significantly improve functional outcomes in patients with nerve injuries post-thyroid surgery, highlighting the value of integrated care pathways [41].
Looking ahead, ongoing research is focused on further enhancing nerve preservation and recovery in thyroid surgery. Regenerative medicine, including stem cell therapies and growth factor treatments, holds promise for facilitating nerve regeneration and functional recovery following injury [37]. Advances in neurophysiological monitoring tools aim to develop more sensitive and specific methods for early detection of nerve stress, thereby enabling even more timely and effective interventions [38]. Personalized surgical planning, utilizing patient-specific anatomical models and predictive analytics, seeks to tailor surgical approaches to individual patient anatomy, thereby minimizing the risk of nerve injury [39]. Long-term outcome studies are also essential for better understanding recovery trajectories and optimizing rehabilitation protocols, ensuring that patients receive the most effective and evidence-based care [40].
In conclusion, managing suspected nerve injury during thyroid surgery requires a comprehensive and multidisciplinary approach that integrates preventive strategies, real-time monitoring, prompt surgical interventions, and robust postoperative care. Advances in technology, combined with continuous education and multidisciplinary collaboration, are pivotal in minimiz-ing the incidence and impact of nerve injuries, ultimately enhancing patient outcomes and quality of life. Future innovations in regenerative medicine and personalized surgical planning hold significant promise for further advancements in nerve injury management, paving the way for safer and more effective thyroid surgical procedures.
Notes
Funding
None.
Conflict of Interest
Seung Hoon Woo is the Editor-in-Chief of the journal, but was not involved in the review process of this manuscript. Otherwise, there is no conflict of interest to declare.
Data Availavility
None.