| J Neuromonit Neurophysiol > Volume 4(2); 2024 > Article |
|
Abstract
Intraoperative neuromonitoring (IONM) has become an indispensable component in thyroid and head and neck surgeries, significantly enhancing surgical precision and patient safety. This comprehensive review explores the latest advancements in IONM techniques, emphasizing developments in both clinical and basic research. Additionally, it integrates perspectives from regenerative medicine, highlighting how emerging therapies can synergize with neuromonitoring to optimize nerve preservation and recovery. We examine the evolution of neuromonitoring technologies, their application in various surgical procedures, and the collaborative efforts across disciplines that have enhanced neuromonitoring efficacy. Furthermore, the review addresses the impact of IONM on surgical strategies, discusses current challenges, and outlines future directions in the field, including the potential integration of regenerative medicine approaches. By synthesizing current knowledge, this paper aims to provide a valuable resource for clinicians and researchers striving to optimize surgical outcomes through effective neuromonitoring and regenerative strategies.
Thyroid and head and neck surgeries, while generally safe and effective, inherently carry significant risks to neural structures. The recurrent laryngeal nerve (RLN) and the superior laryngeal nerve (SLN) are particularly vulnerable during these procedures [1]. Injury to these nerves can lead to vocal cord paralysis, dysphagia, aspiration, and other complications that severely impact patients' quality of life. Intraoperative neuromonitoring (IONM) has emerged as a critical tool in mitigating these risks by providing real-time feedback on neural function during surgery, thereby enhancing surgical precision and patient safety [2,3].
IONM encompasses a range of techniques aimed at monitoring the functional integrity of neural pathways throughout surgical procedures. Its application in thyroid and head and neck surgeries has evolved markedly, driven by technological advancements and a deeper understanding of neurophysiological principles [3]. This paper aims to consolidate current advancements in IONM techniques, assess their clinical applications, and explore the collaborative efforts across disciplines that have enhanced neuromonitoring efficacy. Additionally, it delves into the intersection of IONM with regenerative medicine, examining how emerging regenerative therapies can complement neuromonitoring to promote nerve preservation and recovery. By integrating both clinical and basic research perspectives, this paper seeks to highlight the multifaceted role of IONM in optimizing surgical outcomes and encouraging multidisciplinary research synergies.
The concept of neuromonitoring during surgery dates back to the mid-20th century, initially applied in neurosurgical procedures to monitor the functional integrity of neural pathways. Early applications of neuromonitoring in thyroid and head and neck surgeries emerged in the late 20th century, driven by the imperative to reduce iatrogenic nerve injuries. Initially, IONM relied heavily on electromyography (EMG) to detect nerve activity, enabling surgeons to identify and preserve neural structures during surgery [4]. These pioneering methods, while groundbreaking, were constrained by technological limitations and the absence of standardized protocols.
In the 1980s and 1990s, the introduction of intermittent IONM in thyroid surgeries marked a significant milestone. Surgeons began to adopt standardized protocols for nerve monitoring, which contributed to a notable decrease in RLN injury rates [4]. The integration of IONM into routine surgical practice was facilitated by advancements in EMG technology and the establishment of multidisciplinary teams comprising surgeons and neurophysiologists. This period laid the foundation for subsequent innovations in neuromonitoring techniques, setting the stage for more sophisticated and continuous monitoring systems that would further enhance surgical outcomes.
Significant technological advancements have transformed IONM from a primarily diagnostic tool to an integral component of surgical practice. The development of continuous intraoperative neuromonitoring (cIONM) has provided ongoing neural feedback throughout the surgical procedure, as opposed to intermittent monitoring, which offers only periodic assessments [5]. High-definition EMG systems have improved signal clarity and accuracy, enabling more precise nerve identi-fication and monitoring. These advancements have enhanced the sensitivity and specificity of neuromonitoring, allowing for earlier detection of potential nerve injuries [6].
Moreover, the integration of video-laryngoscopy has enhanced visualization of vocal cord function, complementing EMG data and providing a comprehensive assessment of nerve integrity. Automated nerve identification systems, incorporating algorithms that differentiate nerve signals from surrounding tissues, have further refined the monitoring process. The incorporation of artificial intelligence (AI) and machine learning algorithms holds promise for enhancing signal interpretation and predictive analytics in real-time, potentially forecasting nerve injury before it occurs and allowing for immediate corrective actions [7]. These technological innovations have collectively contributed to the increased reliability and efficacy of IONM, making it an indispensable tool in modern thyroid and head and neck surgeries. Additionally, the intersection with regenerative medicine technologies, such as nerve growth factors and stem cell therapy, presents new avenues for enhancing nerve preservation and recovery during and after surgery. Figure 1 depicts the setup for IONM in modern surgical environments.
Thyroidectomy, the surgical removal of all or part of the thyroid gland, is one of the most common endocrine surgeries performed worldwide. The primary application of IONM in thyroid surgery is the identification and preservation of the RLN and SLN. Injury to the RLN can result in vocal cord paralysis, leading to voice changes, while damage to the SLN can impair pitch control and vocal endurance [9]. These complications can significantly diminish patients' quality of life, underscoring the importance of effective neuromonitoring.
IONM significantly reduces the incidence of RLN and SLN injuries, particularly in reoperative surgeries where scar tissue and altered anatomy increase the risk of nerve damage. By providing real-time identification of nerves, IONM enables surgeons to navigate around them more safely, thereby minimizing the likelihood of inadvertent trauma. Additionally, the use of IONM has facilitated the adoption of minimally invasive and robotic thyroidectomies by providing clear neural landmarks and real-time functional assessment, enhancing both the safety and efficacy of these advanced surgical methods [10]. Furthermore, integrating regenerative medicine approaches, such as the application of nerve growth factors during thyroid surgery, can be monitored and optimized using IONM to promote nerve healing and functional recovery postoperatively.
Head and neck surgeries, including parotidectomy, laryngeal surgery, and skull base approaches, involve complex anatomy with multiple cranial nerves at risk. IONM plays a crucial role in preserving cranial nerves involved in motor and sensory functions, thereby minimizing postoperative deficits. For instance, during parotid gland removal, the facial nerve is particularly vulnerable. IONM assists in identifying and preserving the facial nerve, reducing the risk of facial paralysis and improving postoperative functional outcomes [11].
In laryngeal surgeries, procedures involving the larynx carry the risk of injuring the RLN and SLN, impacting vocal function. IONM facilitates the precise dissection and preservation of these nerves, ensuring better postoperative vocal outcomes. Techniques such as videolaryngoscopy combined with EMG monitoring have enhanced the accuracy of nerve preservation [12]. Furthermore, in skull base surgeries, which often involve the proximity of multiple cranial nerves, IONM enables continuous monitoring of these nerves, allowing surgeons to navigate safely through intricate anatomical pathways. The use of multimodal IONM, combining EMG, somatosensory evoked potentials, and motor evoked potentials, has further improved the preservation of neural function in these high-risk surgeries [13]. Integrating regenerative medicine strategies, such as the application of stem cell therapies or neurotrophic factors during head and neck surgeries, can be monitored via IONM to assess their efficacy in promoting nerve regeneration and functional recovery [13]. Figure 2 depicts one of the modern IONM applications during surgery.
IONM has revolutionized nerve identification techniques by providing both visual and functional confirmation of nerve integrity. Traditional nerve identification relies heavily on anatomical landmarks and the surgeon’s experience, which can be challenging in cases with distorted anatomy or reoperative fields [15]. IONM enhances this process by allowing for real-time electrical stimulation and monitoring of nerve responses, thereby increasing the precision of nerve identification and preservation.
The use of stimulating probes and feedback mechanisms enables direct electrical stimulation of nerves, eliciting EMG responses that confirm nerve identity and functionality. This approach reduces the reliance on anatomical landmarks alone and increases the accuracy of nerve identification. Additionally, visual confirmation techniques such as video-laryngoscopy complement EMG data by providing real-time visualization of vocal cord movement, further ensuring the functional integrity of the RLN and SLN [16]. The integration of advanced imaging modalities, such as intraoperative ultrasound and neuronavigation systems, with IONM allows for precise localization of nerves within complex anatomical regions [17]. This synergy between imaging and neuromonitoring facilitates safer and more effective surgical dissections, particularly in anatomically challenging or previously operated areas.
Furthermore, the incorporation of regenerative medicine strategies, such as the application of nerve growth factors or stem cell therapies, can be monitored using IONM to assess their efficacy in promoting nerve regeneration and functional recovery [18]. By providing real-time feedback on nerve function, IONM can guide the application and optimization of regenerative treatments, ensuring that therapeutic interventions are both timely and effective. For example, the administration of neurotrophic factors can be synchronized with nerve monitoring data to maximize their regenerative potential, thereby enhancing postoperative neural outcomes [19].
Real-time neural feedback from IONM significantly influences surgical decision-making and strategy. The ability to assess nerve function intraoperatively allows surgeons to make informed decisions regarding the extent of tissue resection, the necessity for nerve grafting, and other critical aspects of the procedure. For instance, during thyroidectomy, IONM data can help determine the optimal extent of tissue removal by providing information on nerve proximity and function, thereby balancing complete tumor resection with nerve preservation [20].
IONM also enables the assessment of nerve viability by evaluating the integrity of neural signals. If IONM indicates compromised nerve function, surgeons can adjust their technique, employ nerve-sparing methods, or consider intraoperative nerve grafting to preserve function. Continuous feedback from IONM allows for intraoperative adjustments to the surgical approach, such as modifying dissection techniques or applying protective measures when a decrease in nerve signal amplitude is detected [21]. This dynamic interplay between monitoring and surgical strategy underscores the importance of IONM in tailored surgical approaches, ultimately optimizing surgical outcomes and minimizing postoperative complications [22].
Moreover, the integration of regenerative medicine approaches can be informed by IONM data. For example, if IONM detects early signs of nerve stress or injury, regenerative therapies such as the application of neurotrophic factors or stem cells can be initiated intraoperatively to support nerve recovery [23]. This proactive approach leverages neuromonitoring data to enhance the effectiveness of regenerative treatments, promoting better functional outcomes for patients. Additionally, the timing and dosage of regenerative agents can be optimized based on real-time monitoring, ensuring that therapeutic interventions are both effective and minimally invasive.
Clinical trials play a crucial role in evaluating the efficacy and outcomes associated with IONM in thyroid and head and neck surgeries. Table 1 summarizes key clinical trials that have assessed various aspects of IONM, including its impact on nerve injury rates, functional outcomes, and the integration of advanced technologies such as AI and multimodal monitoring systems.
For instance, Bai et al. conducted a multicenter randomized controlled trial (RCT) involving 500 patients undergoing thyroidectomy, comparing outcomes between those who received IONM and those who did not. The study found that the IONM group had a significantly lower RLN injury rate (2% vs 5%, p < 0.01), underscoring the protective role of neuromonitoring in reducing nerve damage during thyroid surgeries [27]. Additionally, Yu et al. performed an RCT with 150 patients to compare continuous versus intermittent IONM in thyroidectomy, revealing that the continuous IONM group showed better preservation of vocal cord function (90% vs. 75%, p < 0.05) [28]. These findings highlight the critical role of IONM in enhancing surgical outcomes and suggest potential synergies with regenerative therapies aimed at nerve repair and regeneration.
Prospective cohort studies, such as the one by Staubitz et al., involving 200 patients undergoing head and neck surgeries with continuous IONM, reported a 15% improvement in cranial nerve preservation rates compared to intermittent IONM [26]. Systematic reviews, including those by Cozzi et al. and Skarp et al., have consistently demonstrated that the use of IONM reduces overall nerve injury rates and improves functional outcomes across various surgical contexts [24,25].
Advanced technological integrations have also been evaluated in clinical trials. Isikay et al. conducted a retrospective analysis of 350 surgeries using AI-integrated IONM systems, finding that these systems predicted nerve injury with 85% accuracy, enabling preventive measures [24]. These clinical trials collectively provide robust evidence supporting the use of IONM in enhancing surgical outcomes. The consistent reduction in nerve injury rates, improvement in functional outcomes, and successful integration of advanced technologies underscore the critical role of IONM in modern thyroid and head and neck surgeries. Moreover, the potential integration of regenerative medicine approaches, such as the application of neurotrophic factors during surgery, can be monitored using IONM to assess their efficacy in promoting nerve regeneration and functional recovery, thereby further enhancing patient outcomes.
The integration of IONM into thyroid and head and neck surgeries represents a significant advancement in surgical practice, with substantial evidence supporting its efficacy in reducing nerve injury rates and improving patient outcomes. The clinical trials summarized in Table 1 demonstrate that IONM not only enhances the surgeon's ability to identify and preserve critical neural structures but also facilitates the adoption of advanced surgical techniques such as minimally invasive and robotic approaches. These findings highlight the importance of IONM as a standard component of surgical protocols in these high-risk procedures.
One of the key strengths of IONM is its ability to provide real-time feedback, enabling surgeons to make immediate adjustments to their technique to prevent nerve damage [29]. This dynamic monitoring capability is particularly valuable in complex and reoperative surgeries where anatomical variations and scar tissue can obscure nerve pathways. Furthermore, the integration of cIONM has extended the monitoring period, offering ongoing neural feedback that enhances the precision of nerve preservation throughout the entire surgical procedure [30].
The multidisciplinary collaboration between surgeons, neurophysiologists, and technologists has been instrumental in advancing IONM techniques and technologies. This collaborative approach has facilitated the development of standardized protocols, ensuring consistency and reliability across different surgical settings. Additionally, the incorporation of AI and machine learning algorithms into IONM systems represents a frontier of innovation that holds promise for further improving the predictive accuracy and functionality of neuromonitoring tools. These technological integrations not only enhance signal interpretation but also provide predictive analytics that can foresee potential nerve injuries, allowing for preemptive interventions.
Moreover, the intersection of IONM with regenerative medicine presents exciting opportunities for enhancing nerve preservation and recovery. Regenerative therapies, such as the application of nerve growth factors, stem cell treatments, and bioengineered nerve conduits, can be synergistically integrated with IONM to promote nerve regeneration and functional recovery postoperatively. IONM can monitor the efficacy of these regenerative treatments in real-time, providing valuable feedback that can guide the optimization of therapeutic interventions. For instance, if IONM detects early signs of nerve stress or injury, regenerative therapies can be initiated intraoperatively to support nerve healing, thereby improving long-term functional outcomes for patients.
However, despite the demonstrated benefits, the implementation of IONM is not without challenges. Technical limitations such as signal interference, equipment sensitivity, and the need for standardized protocols remain areas requiring ongoing research and development. Additionally, the proficiency of surgical and neurophysiological teams in utilizing IONM techniques is paramount to achieving optimal outcomes. Comprehensive training and education programs are essential to ensure that practitioners are well-versed in the use of IONM equipment and the interpretation of neural signals.
Future research should focus on refining neuromonitoring technologies, exploring novel biomarkers for nerve function, and integrating AI-driven analytics for predictive monitoring. Technological refinements aimed at enhancing the sensitivity, specificity, and user-friendliness of IONM devices are crucial for improving the practicality and effectiveness of neuromonitoring. Innovations such as wireless monitoring systems, portable IONM units, and advanced signal processing algorithms hold promise for enhancing the accessibility and functionality of IONM.
Exploring novel biomarkers for nerve function could provide additional layers of information for IONM, enhancing its predictive capabilities. Biomarkers that reflect nerve viability, inflammation, or regeneration could offer insights into the real-time status of neural structures, enabling more precise monitoring and intervention strategies. Additionally, the integration of AI and machine learning into IONM systems presents opportunities for developing predictive models that can anticipate nerve injury based on real-time data. These intelligent monitoring systems could provide actionable insights to surgeons during procedures, further reducing the risk of nerve damage.
The intersection with regenerative medicine opens new research avenues, such as the development of IONM-guided regenerative therapies. Research into the optimal timing, dosage, and delivery methods for regenerative agents can be enhanced through real-time neuromonitoring data, ensuring that therapeutic interventions are both effective and minimally invasive. Furthermore, studying the long-term outcomes of combining IONM with regenerative treatments will provide valuable insights into the sustained benefits of such integrated approaches. Collaborative studies involving neurosurgeons, regenerative medicine specialists, and bioengineers will be pivotal in advancing this interdisciplinary field.
In conclusion, IONM has established itself as a critical tool in thyroid and head and neck surgeries, significantly contributing to the reduction of nerve injury rates and the improvement of patient functional outcomes. The continued advancements in IONM technologies, coupled with multidisciplinary research and collaboration, are poised to further enhance the efficacy and reliability of neuromonitoring practices. Integrating regenerative medicine approaches with IONM offers a promising avenue for optimizing nerve preservation and promoting functional recovery, thereby advancing the field of surgical neurophysiology. Addressing existing challenges through technological innovation and comprehensive training will ensure that IONM remains at the forefront of surgical excellence, ultimately leading to superior patient care and outcomes.
IONM has significantly advanced thyroid and head and neck surgeries by enhancing nerve preservation and improving patient outcomes. Technological innovations, including the integration of AI-driven analytics, have transformed IONM into a critical component of modern surgical strategies. In thyroid surgeries, IONM has notably reduced recurrent and superior laryngeal nerve injuries, especially in complex and reoperative cases. Similarly, in head and neck surgeries, IONM has been indispensable in preserving cranial nerves, thereby minimizing postoperative deficits.
Collaborative efforts among surgeons, neurophysiologists, and engineers have driven the refinement and expansion of IONM applications. Additionally, integrating regenerative medicine with IONM offers promising opportunities for enhancing nerve preservation and promoting functional recovery through therapies such as neurotrophic factors and stem cell treatments.
Despite its benefits, challenges like technical limitations, the need for standardized protocols, and comprehensive training remain. Addressing these through ongoing research, technological advancements, and education will further establish IONM as a standard in neurosurgical procedures. Future research directions, including AI integration, novel biomarkers, and predictive monitoring systems, hold potential for even greater advancements, ensuring that IONM continues to enhance surgical outcomes and patient care.
References
1. Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg 2014;22:439-43.


2. Wanjari M, Mittal G, Prasad R. Intraoperative neurophysiological monitoring: a vital tool for surgical precision and patient safety. Neurosurg Rev; 2024. 47:p.550.
3. Guzzi G, Ricciuti RA, Torre AD, Turco EL, Lavano A, Longhini F, et al. Intraoperative neurophysiological monitoring in neurosurgery. J Clin Med 2024;13:2966.



4. Wojtczak B, Sutkowska-Stepien K, Glod M, Kaliszewski K, Sutkowski K, Barczynski M. Current knowledge on the use of neuromonitoring in thyroid surgery. Biomedicines 2024;12:675.



5. Noda T, Ishisaka T, Okano K, Kobayashi Y, Shimode Y, Tsuji H. Experience with the use of intraoperative continuous nerve monitoring in video-assisted neck surgery and external cervical incisions. Laryngoscope Investig Otolaryngol 2021;6:346-53.




6. Sung E, Lee J, Shin S, Kwon H, Kim M, Kim D, et al. Development of a novel intraoperative neuromonitoring system using a surface pressure sensor to detect muscle movement: a rabbit model study. Clin Exp Otorhinolaryngol 2019;12:217-23.



7. Wang C, He T, Zhou H, Zhang Z, Lee C. Artificial intelligence enhanced sensors - enabling technologies to next-generation healthcare and biomedical platform. Bioelectron Med 2023;9:17.




8. Park D, Kim I. Application of machine learning in the field of intraoperative neurophysiological monitoring: a narrative review. Applied Sciences 2022;12:7943.

9. Ryu CH, Lee SJ, Cho J, Choi IJ, Choi YS, Hong YT, et al. Care and management of voice change in thyroid surgery: Korean society of laryngology, phoniatrics and logopedics clinical practice guideline. Clin Exp Otorhinolaryngol 2022;15:24-48.



10. Park J, Kim K. Current and future of robotic surgery in thyroid cancer treatment. Cancers (Basel) 2024;16:2470.



11. Turhal G, Hepkarsi S, Ozturk K. The potential applicability of facial nerve monitoring as a navigation tool in parotid gland surgery. Braz J Otorhinolaryngol 2023;89:230-4.


12. Ghatol D, Widrich J. Intraoperative neurophysiological monitoring, in StatPearls. StatPearls Publishing; 2024.
13. Rajappa D, Khan MM, Masapu D, Manchala R, Rudrappa S, Gopal S, et al. Multimodal intraoperative neurophysiological monitoring in Spine surgeries: the experience at a spine centre through years. Asian Spine J 2021;15:728-38.



14. Daroszewski P, Huber J, Kaczmarek K, Janusz P, Glowka P, Tomaszewski M, et al. “Real-time neuromonitoring” increases the safety and non-invasiveness and shortens the duration of idiopathic scoliosis surgery. J Clin Med 2024;13:1497.



15. Starnoni D, Giammattei L, Cossu G, Link MJ, Roche P, Chacko AG, et al. Surgical management for large vestibular schwannomas: a systematic review, metaanalysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien) 2020;162:2595-617.




16. Chilkoti GT, Gupta A, Bhandari P, Mohta M. Techniques of detecting recurrent laryngeal nerve palsy in patients undergoing thyroid surgery: pearls and pitfalls. J Anaesthesiol Clin Pharmacol 2024;40:199-205.



17. Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 2012;9:491-500.



18. Li R, Li D, Zhang H, Wang J, Li X, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin 2020;41:1289-300.




19. Houlton J, Abumaria N, Hinkley SFR, Clarkson AN. Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front Neurosci 2019;13:790.



20. Choi SY, Son YI. Intraoperative neuromonitoring for thyroid surgery: the proven benefits and limitations. Clin Exp Otorhinolaryngol 2019;12:335-6.




21. Mathieson T, Jimaja W, Triponwz F, Karenovics W, Makovac P, Muradbegovic M, et al. Safety of continuous intraoperative vagus nerve neuromonitoring during thyroid surgery. BJS Open 2023;7:zrad039.




22. Wilson JP, Vallejo JB, Kumbhare D, Guthikonda B, Hoang S. The use of intraoperative neuromonitoring for cervical spine surgery: indications, challenges, and advances. J Clin Med 2023;12:4652.



23. Chu X, Song X, Li Q, Li Y, He F, Gu X, et al. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen Res 2022;17:2185-193.



24. Skrap B, Bonaventura RD, Domenico MD, Sturiale CL, Auricchio AM, Maugeri R, et al. Has intraoperative neuromonitoring changed the surgery for unruptured middle cerebral artery aneurysms? A retrospective comparative study. Neurosurg Rev 2023;46:191.




25. Cozzi AT, Ottavi A, Lozza P, Maccari A, Borloni R, Nitro L, et al. Intraoperative neuromonitoring does not reduce the risk of temporary and definitive recurrent laryngeal nerve damage during thyroid surgery: a systematic review and meta-analysis of endoscopic findings from 73,325 nerves at risk. J Pers Med 2023;13:1429.



26. Staubitz JI, Musholt TJ. Continuous intraoperative recurrent laryngeal nerve monitoring: techniques, applications, and controversies. Curr Otorhinolaryngol Rep 2021;9:326-33.


27. Bai B, Chen W. Protective effects of intraoperative nerve monitoring (IONM) for recurrent laryngeal nerve injury in thyroidectomy: meta-analysis. Sci Rep 2018;8:7761.




28. Yu Q, Liu K, Zhang S, Li H, Xie C, Wu Y, et al. Application of continuous and intermittent intraoperative nerve monitoring in thyroid surgery. J Surg Res 2019;243:325-31.


Figure 1.
Schematic illustration of intraoperative neurophysiological monitoring (IONM). A multidisciplinary approach between the surgeon, physiatrist, and anesthesiologist is necessary throughout the process. Several confounding factors, such as surgical, anesthesiologic, and mechanical factors, as well as the patient’s condition and inter-rater variability complicate the interpretation of IONM. Reused from the article of Park et al. (Applied Sciences 2022;12:7943) [8].
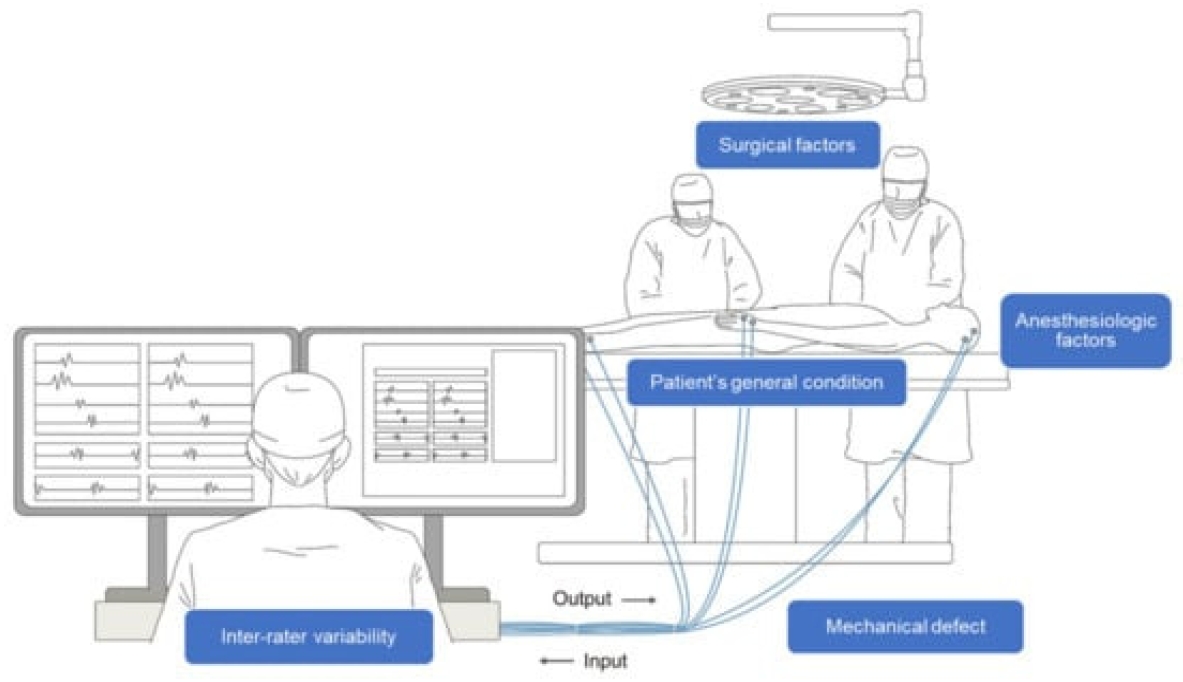
Figure 2.
Methodological principles of neurophysiological recordings and study design. The same neurophysiological methodology was used in healthy volunteers once. (A) Location of recording bipolar electrodes over anatomical passage of peroneal nerve (PER) and surface of tibialis anterior (TA) muscle applied in pre- and postoperative examinations. (B) Preoperative positioning of stimulating coil over scalp for transcranial magnetic stimulation (TMS). Experimentally changed following tracking with aim of obtaining best highest-amplitude motor evoked potential (MEP) recordings, which allowed marking black points of hot spots. (C) Intraoperative stimulating electrode application. (D) Placement of intraoperative recording bipolar electrodes over TA muscle and PER nerve, as well as rectus femoris (RF) muscle. (F) Transcranial electrical stimulation (TES). (E) It was placed over the forearm extensor carpi (EXTC) muscle group and stabilized with adhesive patch tapes. (G) Prone position of patient with applied recording electrodes prepared for surgery from back approach in theater. (H) Photographs illustrating view of thoracolumbar spine preparation at subsequent steps before, (K) and after implantation of two titanium rods for distraction and derotation procedures performed by surgeon. (I) Positioning of transpedicular screws was also verified via X-rays with C-arm. (J) Neuromonitoring recordings in distant range from surgical field aimed at verifying spinal neural motor transmission without, in “Interactive S-N” patient group or with, (L) in “Real-time” patient group camera picture support and simultaneous MEP recordings. Reused from the article of Daroszewski et al. (J Clin Med 2024;13:1497) [14].
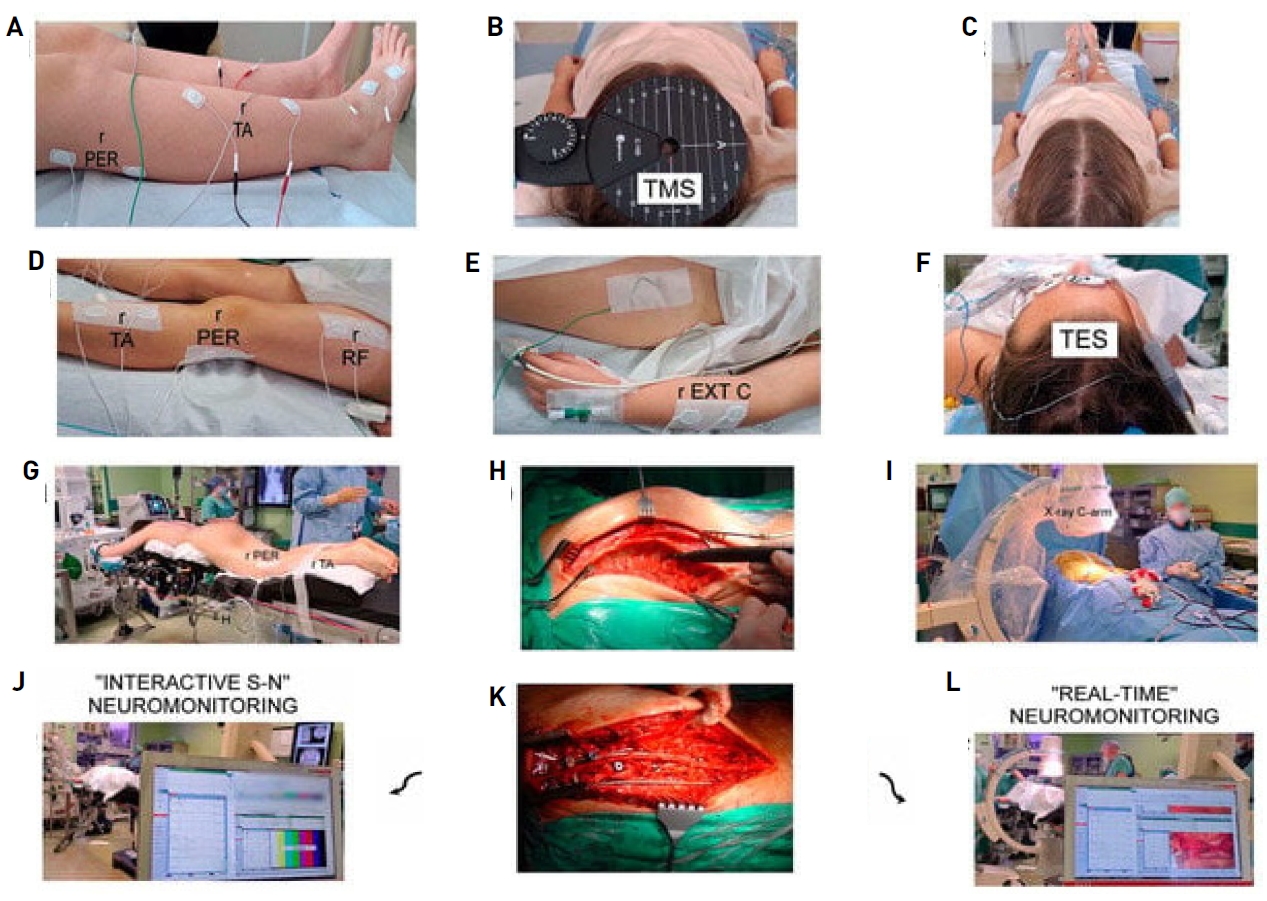
Table 1.
Clinical trials evaluating intraoperative neuromonitoring
| Design | Year | Intervention | Outcome Measures | Key Findings | Ref. |
|---|---|---|---|---|---|
| Retrospective cohort | 2023 | IONM-assisted vs non-IONM surgery for MCA aneurysms | Functional outcomes, ischemia, aneurysmal remnants | IONM improved functional outcomes and reduced ischemic complications (p < 0.05). | [24] |
| Systematic review | 2023 | Comparison of IONM (intermittent/continuous) to non-IONM in thyroidectomy | RLN injury rates (temporary/permanent) | IONM showed no significant reduction in RLN injuries but aided complex surgeries. | [25] |
| Review | 2021 | Continuous IONM (cIONM) | Vocal cord palsy rates | cIONM reduced transient RLN injuries compared to intermittent techniques. | [26] |
| Retrospective cohort | 2018 | IONM vs non-IONM in thyroidectomy | RLN injury prevention | IONM significantly decreased transient RLN injuries (RR = 0.71, CI 0.57 - 0.88). | [27] |
| Multicenter cohort studies | 2019 | IONM for thyroid malignancies and goiters | Nerve identification and preservation rates | Enhanced identification and reduced transient injuries in complex surgeries. | [28] |
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 658 View
- 9 Download
- ORCID iDs
- Related articles
-
Intraoperative neuromonitoring during robotic thyroidectomy2025 November;5(2)
Recent studies of intraoperative neuromonitoring during thyroid surgery2023 November;3(2)
Practical application of neuromonitoring using skin electrodes in thyroidectomy2023 November;3(2)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print