Precision and protection: advancements and future of neuromonitoring techniques for laryngeal surgery
Article information
Abstract
Intraoperative neuromonitoring (IONM) has improved the safety of laryngeal surgeries by protecting critical nerves such as the recurrent laryngeal nerve and vagus nerve. This review traces the evolution of neuromonitoring from its late 19th-century origins to the modern era, focusing on the transition from intermittent intraoperative neuromonitoring (I-IONM) to continuous intraoperative neuromonitoring (cIONM). Unlike I-IONM, which offers periodic nerve activity snapshots, cIONM provides continuous, real-time feedback throughout surgery, improving nerve injury detection and prevention. Advances in electromyography (EMG) techniques and the laryngeal adductor reflex have been key in this progression. The integration of endotracheal tube-based systems has revolutionized monitoring by providing less invasive and more accurate assessments. The review also explores cIONM's role in minimally invasive surgeries, such as robotic and endoscopic procedures, offering enhanced precision and better patient outcomes. It further discusses recent advancements in EMG techniques and future directions, highlighting the potential for innovations to enhance the precision and safety of laryngeal surgeries.
Introduction
Intraoperative neuromonitoring (IONM) has seen substantial evolution over the past century, becoming a cornerstone of modern surgery. Its primary aim is to monitor neural structures' functional integrity during operations, thereby preventing neurological deficits. This technology is especially beneficial in surgeries involving the spine, brain, and peripheral nerves. The origins of neuromonitoring date back to the late 19th century with the initial use of electrical stimulation for neural mapping [1,2]. Significant advancements came in the mid-20th century with the development of electromyography (EMG) and evoked potentials (EPs), which formed the basis of modern neuromonitoring. EMG captures muscle electrical activity, while EPs measure the brain's response to sensory stimuli. Intermittent intraoperative neuromonitoring (I-IONM) emerged from these technologies, involving periodic nerve function assessments at specific surgery intervals [3-5].
IONM has revolutionized laryngeal surgeries, enhancing safety and outcomes by protecting critical neural structures, specifically the recurrent laryngeal nerve (RLN) and the vagus nerve [1,2,6,7]. These nerves are essential for voice, swallowing, and breathing functions, making their preservation during procedures like thyroidectomy, parathyroidectomy, and laryngectomy crucial. The RLN, innervating the vocal cords, is especially vulnerable due to its anatomical path. Injury to this nerve can cause vocal cord paralysis, leading to hoarseness, voice loss, and severe airway complications. Real-time monitoring of neural function via IONM is vital to avoid such adverse outcomes [1,7].
Despite its usefulness, I-IONM had the drawback of providing only momentary neural activity snapshots, potentially missing transient changes indicating nerve injury [8-10]. The shift from intermittent to continuous intraoperative neuromonitoring (cIONM) was transformative. cIONM provides real-time, ongoing nerve function assessments throughout surgery, allowing immediate detection and correction of any nerve activity changes to prevent permanent damage. Key advancements driving cIONM's development and adoption include refined EMG techniques, which offer constant muscle activity data, and the introduction of sophisticated monitoring equipment and software that enhance cIONM's sensitivity and accuracy [11-14].
Technical Aspects and Success of cIONM in Laryngeal Surgery
cIONM has become an essential tool in modern surgical practices, especially in procedures where the protection of neural structures is critical. The primary aim of cIONM is to provide real-time, continuous feedback on the functional integrity of nerves during surgery, thereby minimizing the risk of nerve damage and improving patient outcomes [8-10]. At its core, cIONM involves the continuous recording of EMG signals from muscles that are innervated by the nerves at risk. This real-time monitoring allows for the immediate detection of any changes in nerve activity, which can indicate potential nerve damage. The continuous nature of this monitoring provides a constant stream of data, enabling the surgical team to take prompt corrective actions if any abnormalities are detected [13,14]. This is particularly useful in surgeries involving the RLN and the vagus nerve, which are critical for functions such as speech and swallowing. Although continuous IONM may incur higher costs, its advantages in minimizing surgical complications significantly outweigh the financial implications. By providing real-time monitoring of nerve function, continuous IONM empowers surgeons to make informed decisions and take immediate corrective actions, ultimately leading to improved patient outcomes and reduced postoperative morbidity [15-17].
While there are studies demonstrating the superiority of continuous IONM over intermittent IONM in preventing vocal cord palsy, specific success rates can vary depending on factors like surgical complexity, surgeon experience, and patient-specific conditions. Schneider et al. found that continuous IONM reduced the early postoperative vocal cord palsy rate by 1.7-fold and the permanent vocal cord palsy rate by 30-fold compared to I-IONM [17]. This suggests a significant improvement in outcomes with continuous monitoring. However, it's essential to note that the absolute risk of vocal cord palsy is relatively low, even with intermittent monitoring. Therefore, the clinical impact of choosing one technique over the other may not be substantial in all cases. Ultimately, the decision to use continuous or intermittent IONM should be made on a case-by-case basis, considering the specific circumstances of each patient and the surgeon's expertise [15,16].
Laryngeal Adductor Reflex cIONM
One innovative technique within cIONM is the utilization of the laryngeal adductor reflex (LAR). LARcIONM involves continuously stimulating and recording reflexive responses from the laryngeal muscles. Surface electrodes placed on the endotracheal tube stimulate the RLN and record the resulting muscle contractions, providing a non-invasive method to monitor nerve function in real-time [11,12,18]. This technique is highly sensitive, detecting even subtle changes in nerve activity, enabling early intervention and reducing the risk of permanent nerve damage. LAR-cIONM leverages the body's natural protective reflex to monitor the integrity of the RLN during laryngeal surgery. By stimulating the laryngeal mucosa, the LAR is triggered, leading to vocal cord contraction. EMG is used to monitor these muscle contractions and detect any changes in nerve function [11,12,19]. Any disruption to the RLN during surgery can be immediately identified, allowing for corrective action and potentially preventing permanent nerve damage. LAR-cIONM has demonstrated significant success in reducing the incidence of postoperative vocal cord paralysis compared to traditional monitoring techniques [11,12-14].
Vagal Nerve Stimulation in cIONM
Vagal nerve stimulation is another critical component of cIONM, especially in surgeries involving the RLN. The vagus nerve is stimulated continuously to elicit responses from the laryngeal muscles [13,20]. This technique helps in identifying the exact location and functional integrity of the RLN during surgery. Continuous stimulation of the vagus nerve provides real-time feedback on nerve function, enabling the surgical team to detect and respond to any changes promptly. Vagal nerve stimulation in cIONM is particularly useful in thyroid and parathyroid surgeries, where the risk of RLN injury is high [20-22]. By continuously monitoring the nerve function, surgeons can make immediate adjustments to their surgical approach to prevent nerve damage. This technique has been shown to reduce the incidence of postoperative vocal cord paralysis and other nerve-related complications [21,23].
Application of Endotracheal Tube-Based Monitoring Systems
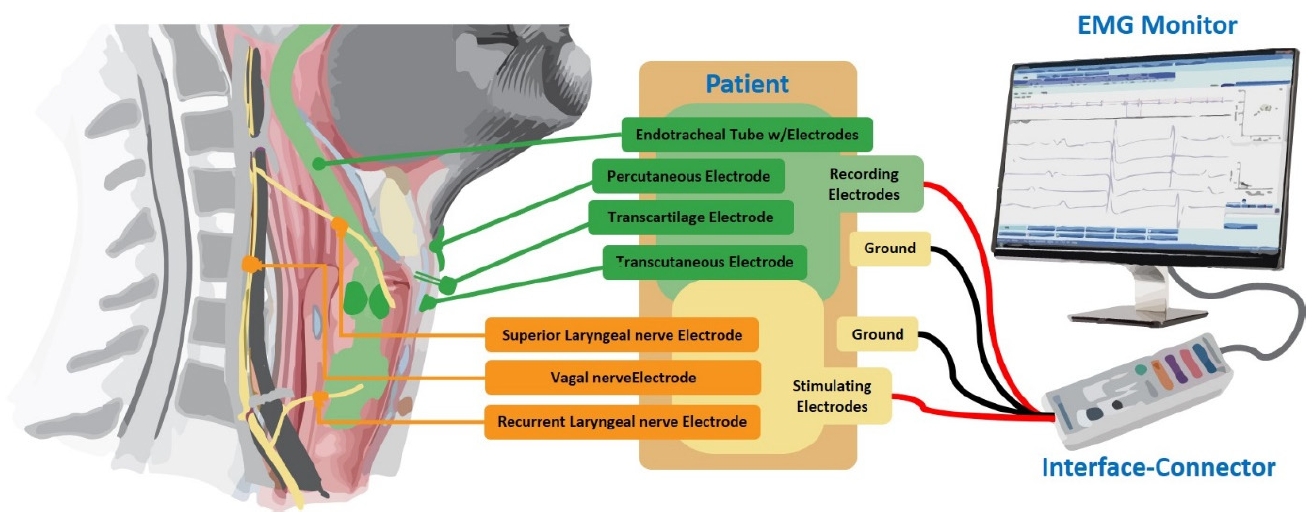
The integration of endotracheal tube-based monitoring systems and cIONM has significantly advanced the precision and protection of laryngeal surgeries. These systems are designed to provide real-time, continuous feedback on nerve function, enhancing the safety and efficacy of surgical procedures [11,12,14]. Endotracheal tube-based monitoring systems are specifically designed to facilitate continuous monitoring of the RLN and other critical neural structures during laryngeal surgeries. By incorporating surface electrodes into the endotracheal tube (ETT), these systems provide a stable and consistent means of recording EMG signals from the laryngeal muscles throughout the surgery [24-27]. This continuous monitoring allows for immediate detection of any changes in nerve activity, enabling prompt corrective actions to prevent nerve damage. Both ETT-based monitoring systems and cIONM provide real-time feedback on nerve function [28-29]. This continuous stream of data allows the surgical team to detect any changes in nerve activity immediately, enabling them to take prompt corrective actions to prevent nerve damage. This is particularly crucial in delicate surgeries involving the larynx, where nerve injury can have significant consequences. ETTbased systems offer a less invasive alternative to traditional needle electrodes, reducing the risk of infection and patient discomfort [25-29]. The surface electrodes on the ETT maintain consistent contact with the laryngeal muscles, ensuring reliable data collection and minimizing the likelihood of signal loss or artifacts. This consistency is vital for effective continuous monitoring. The combination of ETT-based systems and cIONM enhances the precision of neuromonitoring during laryngeal surgeries [2-31]. This increased precision helps in minimizing the risk of postoperative complications such as vocal cord paralysis. By providing continuous, real-time monitoring and reliable feedback on nerve function, the integration of ETT-based systems and cIONM improves overall surgical outcomes [24,25,28,30]. Surgeons can make informed decisions during the procedure, reducing the risk of nerve damage and ensuring better protection of critical neural structures. This results in improved patient safety and postoperative recovery. Endotracheal tube-based monitoring systems have revolutionized IONM, particularly in surgeries involving the laryngeal nerves. These systems integrate surface electrodes onto the ETT, which is used to secure the patient's airway during surgery [29-31]. The electrodes continuously stimulate the nerves and record the resulting muscle activity, allowing real-time monitoring of the RLN and other critical neural structures, providing immediate feedback to the surgical team. Lastly ETT-based systems are easier to set up and use compared to needle electrodes. The integration of electrodes into the ETT streamlines the monitoring process, reducing the complexity and time required for electrode placement. This efficiency is particularly beneficial in high-stakes surgeries where time is critical. ETT-based monitoring systems are compatible with modern surgical techniques, including minimally invasive and robotic surgeries. This compatibility ensures that neuromonitoring can be seamlessly integrated into various surgical approaches, further enhancing the precision and safety of these procedures [24-27]. ETT-based monitoring systems offer significant advantages over traditional needle electrodes, making them a valuable tool in continuous intraoperative neuromonitoring. Their minimally invasive nature, consistent and reliable data collection, ease of use, real-time feedback, and improved patient safety make them an essential component of modern laryngeal surgeries [26,2-30]. These innovations in neuromonitoring technology continue to enhance the quality and outcomes of surgical care. Figure 1 shows a general diagram of how different electrodes are used in IONM for laryngeal surgeries.
Recent Advancements in EMG Techniques and Combined EMG Events for Neuromonitoring in Laryngeal Surgery
EMG plays a pivotal role in cIONM, serving as the primary method for recording and assessing the electrical activity of muscles innervated by nerves at risk during surgery. The relationship between EMG and cIONM is foundational, as EMG provides the real-time data necessary for effective continuous monitoring of neural function. Recent advancements in EMG technology have significantly revolutionized neurophysiology and neuromonitoring fields. One notable development is the advent of wireless and wearable EMG systems [32-35]. These systems offer greater flexibility and convenience compared to traditional wired setups, enabling continuous monitoring of muscle activity. This innovation allows healthcare providers to make more informed decisions about treatment plans and track the progression of neuromuscular disorder. Another significant advancement is the use of flexible noninvasive electrodes for surface EMG acquisition. These electrodes are designed to be more comfortable and less intrusive, making them ideal for long-term monitoring and remote healthcare applications. The improvements in signal processing techniques have also enhanced the accuracy and reliability of EMG data, thereby improving its clinical utility [36-39].
Combining EMG with other neurophysiological techniques, such as electroencephalography (EEG), has further advanced the field. This multimodal approach provides a more comprehensive assessment of neuromuscular function, particularly beneficial in rehabilitative applications. For example, combining EMG and EEG can help evaluate cortico-muscular interactions, offering valuable insights into motor control and rehabilitation outcomes [38,39]. In the realm of surgery, the integration of EMG technology into cIONM has led to the development of advanced monitoring systems that provide quantitative electromyographic data, enabling more precise and reliable assessments of nerve function. These systems can detect adverse EMG events, such as amplitude decreases or latency increases, which indicate potential nerve damage [37,39]. By responding to these events promptly, surgeons can minimize the risk of permanent nerve injury and improve surgical outcomes.
Integration of cIONM in Minimally Invasive Surgical Techniques
cIONM has become a cornerstone in enhancing the precision and safety of robotic and endoscopic laryngeal surgeries [22,40-45]. These minimally invasive techniques require meticulous surgical precision due to the confined and complex anatomy of the laryngeal region, where even slight inaccuracies can lead to significant complications. cIONM plays a critical role in these advanced surgical methods by providing real-time, continuous feedback on the functional integrity of the RLN and other critical neural structures [41,42,46-50].
In robotic and endoscopic surgeries, the surgeon often relies on highly specialized instruments and visual aids, making it crucial to have ongoing, reliable information about nerve function [9,48-52]. cIONM ensures that any potential nerve irritation or damage is detected immediately, allowing the surgical team to take prompt corrective actions. The integration of cIONM with robotic and endoscopic techniques significantly enhances surgical precision. The continuous monitoring of nerve function enables surgeons to navigate the intricate anatomy of the larynx with greater confidence and accuracy. This real-time feedback mechanism helps in avoiding inadvertent nerve injury, which is particularly important in preserving the patient's voice, swallowing function, and overall quality of life.
Furthermore, the use of cIONM in these minimally invasive procedures has been shown to improve surgical outcomes. Studies have demonstrated that continuous monitoring reduces the incidence of postoperative complications such as vocal cord paralysis and other nerve-related dysfunctions [9,40,41,44,49-52]. This translates into faster recovery times, reduced postoperative morbidity, and better overall patient satisfaction. cIONM is indispensable in robotic and endoscopic laryngeal surgeries. Its ability to provide continuous, real-time feedback on nerve function enhances surgical precision and significantly improves outcomes. This advancement not only ensures the safety and efficacy of these complex procedures but also elevates the standard of care in laryngeal surgery, paving the way for more successful and minimally invasive treatment options.
Challenges and Future Directions and Emerging Technologies for cIONM
cIONM has significantly advanced surgical practice, particularly in procedures where protecting neural structures is crucial. However, despite its numerous benefits, cIONM still faces several challenges. Technical challenges include artifact reduction, optimal electrode placement, and accurate signal interpretation [14,53-55]. Clinical challenges encompass standardization of protocols, cost-effectiveness, patient variability, and complex surgical scenarios. To further optimize cIONM, future advancements may involve artificial intelligence (AI)-powered algorithms for improved signal analysis, advanced electrode technologies for enhanced signal quality, and real-time feedback systems integrated with surgical navigation. Addressing these challenges is essential to maximize the benefits of cIONM and improve patient outcomes.
The future of cIONM is poised for significant advancements, driven by emerging technologies and innovative approaches. One of the key areas of development is the integration of AI and machine learning (ML) algorithms into cIONM systems [56-59]. These technologies can enhance the accuracy and efficiency of monitoring by analyzing vast amounts of data in real-time, identifying patterns, and predicting potential complications before they occur [58,59].
Another promising technology is wearable neuroimaging devices. Devices such as EEG [60-63] and functional near-infrared spectroscopy (fNIRS) can continuously monitor brain activity during surgery. These wearable devices offer the advantage of being non-invasive and can provide real-time feedback to surgeons, allowing for immediate adjustments during the procedure [64-66]. Remote monitoring is also gaining traction in the field of cIONM. With the advent of telemedicine, it is now possible to monitor patients remotely, even after they have been discharged from the hospital. This is particularly beneficial for patients who require long-term monitoring and follow-up care. By combining different monitoring techniques, such as EEG, fNIRS, and amplitude-integrated electroencephalography, clinicians can obtain a more comprehensive view of the patient's brain function [60-63]. This approach helps identify subtle changes in brain activity that might be missed by a single monitoring method. Improvements in cIONM methods are expected to enhance both the accuracy and usability of these technologies. One potential improvement is the development of more sophisticated algorithms for data analysis. These algorithms can help reduce false positives and negatives, thereby improving the reliability of cIONM systems (Figure 2).
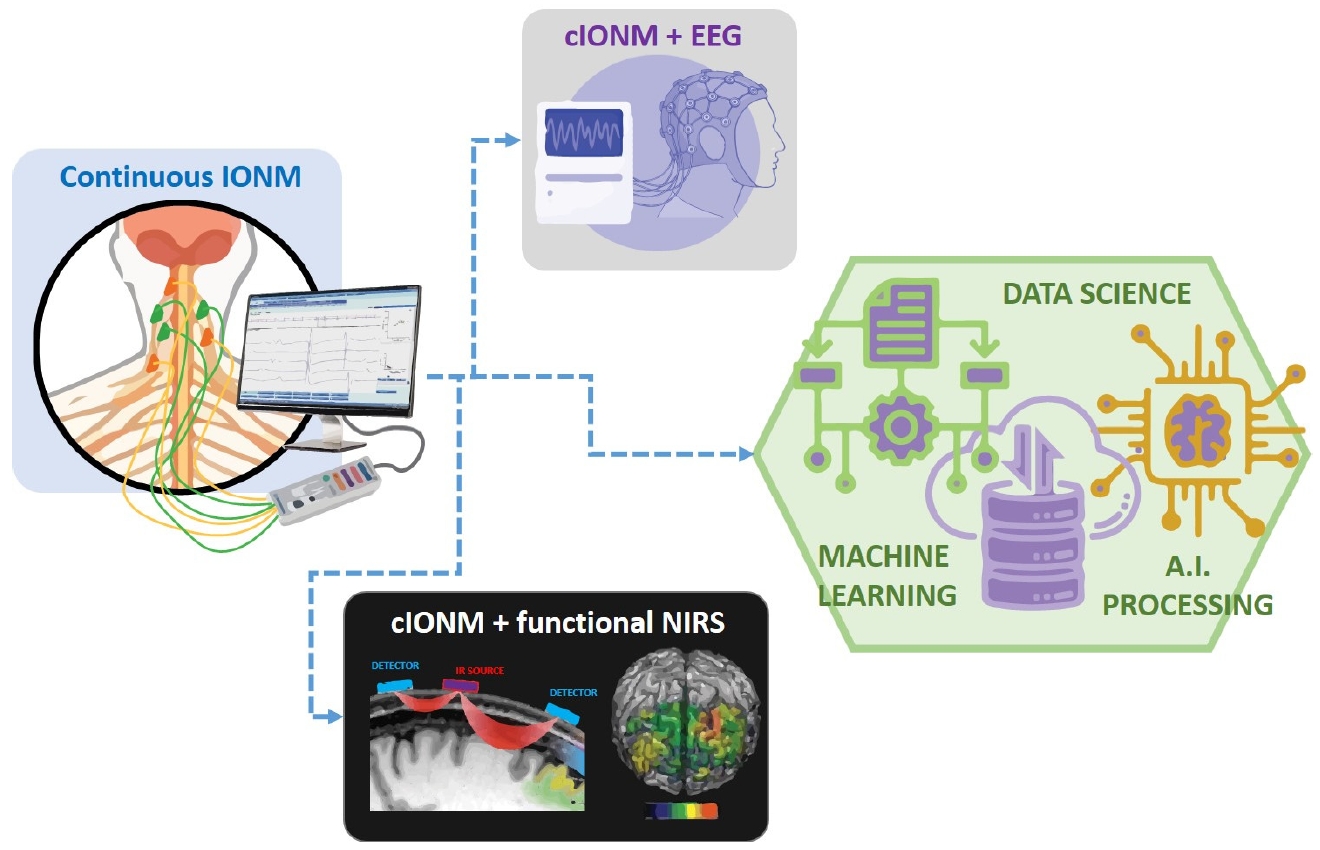
Future directions of cIONM for laryngeal surgeries showing possibilities for combining with EEG, fNIRS, machine learning and artificial intelligence for improving accuracy and advanced monitoring techniques. IONM, intraoperative neuromonitoring; cIONM, continuous intraoperative neuromonitoring; EEG, electroencephalogram; fNIRS, functional near-infrared spectroscopy; AI, artificial intelligence.
Another area of improvement is the miniaturization of monitoring devices. Smaller, more portable devices can be easily integrated into surgical setups, making it easier for surgeons to use them without disrupting the workflow [61,62,64,65,67]. Additionally, advancements in wireless technology can eliminate the need for cumbersome cables, further streamlining the monitoring process. In terms of clinical use, there is a growing emphasis on personalized medicine. By tailoring cIONM protocols to individual patients, clinicians can provide more targeted and effective monitoring. This approach leads to better outcomes and reduces the risk of complications.
Conclusion
In summary, the future of cIONM looks promising with the integration of AI and ML, the use of wearable neuroimaging devices, the adoption of remote and multimodal monitoring, and improvements in data analysis algorithms and device miniaturization. These advancements are set to enhance the precision, reliability, and overall effectiveness of neuromonitoring in clinical settings, leading to improved patient care and surgical outcomes.
Notes
Funding
None.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availavility
None.